Binding
Introduction
Single unit recordings in vivo have revealed much about the events or features to which neurons respond. Individual neurons do not detect their preferred sensory features in isolation; they form part of neuronal networks whose emergent properties define the feature detection properties of the cortical column. In the visual system, it used to be thought that successive hierarchies of neurons encoded progressively more complex features of objects. This scheme, however, is inflexible and inefficient: conjunctions of more and more combinations of "low-level" features are needed to define progressively "higher level" features. It is difficult to see how such a scheme copes with the vast range of objects we can recognize (the "combinatorial problem" [7]), and with the many entirely unprecedented objects found in the modern world. Frisbees, for instance, have no obvious precedent in the world in which our ancestors evolved. (While a recent visit to the Pitt Rivers Museum in Oxford revealed that circular quoits have a long history on the Indian subcontinent as a kind of throwing knife, they were made of metal and not brightly colored plastic!) Yet we are able effortlessly to distinguish a flying Frisbee against a complex background, even though different, widely separated, cortical areas detect its shape, color, movement, etc., and the same areas also detect the features of the background. All this information then can be linked efficiently to the motor system to allow us to catch the Frisbee. A more plausible idea for how we accomplish such tasks is that transient "binding" functionally links the often discontinuous cortical networks needed to analyze the many features that make up real objects [7], and conversely, "scene segmentation" functionally separates the networks encoding different objects from one another and from the background.
The problems of binding, segmentation and the role of synchronous oscillations were clearly identified by von der Malsburg [9] considering the "cocktail party effect", where we can to listen to one speaker despite the background babble. Much of the recent interest in gamma rhythms centers on the visual system [1,3,4,6,7]. Single and multi-unit discharges and local field potentials (Fig. 1A) can be synchronized, at gamma frequencies, between visual cortical areas separated by long distances, as long as those areas were stimulated by, and selective for, the same object. The repeated synchronized firing of neurons in different co-stimulated areas in principle provides a solution to the binding problem. This concept may generalize beyond the specific sensory cortices to association areas and the hippocampus, where information from different sensory modalities converge and may be bound into multimodal entities or perceptions.
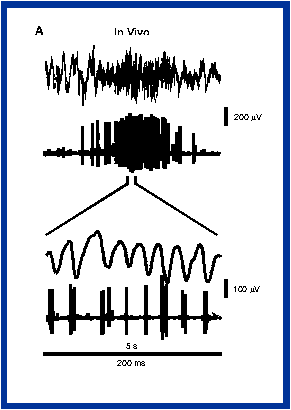
Trends in Neurosciences 19, 202-208.
The original models for binding and segmentation introduced the idea that neurons that oscillated together would work together [9]. Synchronization matters more than tight bandwidth [2,7]. Single episodes of neuronal synchronization might occur by chance, but repeated synchronization is much less likely to do so. At the cellular level repeated synchronization could promote temporal summation at active synapses.
The key point is that the gamma oscillation is not proposed to represent information itself, but rather to provide a temporal structure for correlations in the neurons that do encode specific information [2,5,7]. We speculate that the function of gamma rhythm is to provide a reference clock to control the firing of the excitatory neurons, which do most of the processing of the information in hippocampus and neocortex.
The general significance of this mechanism is controversial. Not everyone finds unit correlations in the gamma band, for instance in some experiments on monkey visual cortex [8,11]. This could be related to choices of stimuli and recording sites, or to technical differences (gamma can occur in brief bursts, with considerable jitter in frequency [2], so it could conceivably be smudged out in averaged measurements on 0.5s runs of EEG, or 20 cycles at 40 Hz [11]). However, the reasons for these discrepancies remain unresolved. Others conclude that while gamma rhythms may exist, they are simply an epiphenomenon; that is, they are just an irrelevant detail that falls out of the need to connect neurons in a particular way [10].
We believe that one key step to resolving these issues is to understand the cellular and network mechanisms that generate gamma rhythms, and to develop pharmacological tools that will allow us to probe their roles in vivo.
Bibliography
1 Engel, A.K., K nig, P., Kreiter, A.K. and Singer, W. Interhemispheric synchronization of oscillatory neuronal responses in cat visual cortex, Science, 252 (1991) 1177-1179.
2 Engel, A.K., K nig, P. and Schillen, T.B. Neural coding: Why does the cortex oscillate, Curr. Biol. 2 (1992) 332-334.
3 Engel, A.K., K nig, P. and Singer, W. Direct physiological evidence for scene segmentation by temporal coding, Proc. Natl. Acad. Sci. USA, 88 (1991) 9136-9140.
4 Freeman, W.J. and van Dijk, B.W. Spatial patterns of visual cortical fast EEG during conditioned reflex in a rhesus monkey, Brain Res. 422 (1987) 267-276.
5 Gray, C.M., Engel, A.K., K nig, P. and Singer, W. Synchronization of oscillatory neuronal responses in cat striate cortex: temporal properties, Vis. Neurosci. 8 (1992) 337-347.
6 Gray, C.M., K nig, P., Engel, A.K. and Singer, W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties, Nature, 338 (1989) 334-337.
7 Singer, W. and Gray, C.M. Visual feature integration and the temporal correlation hypothesis, Annu. Rev. Neurosci. 18 (1995) 555-586.
8 Tovee, M.J. and Rolls, E.T. Oscillatory activity is not evident in the primate temporal visual cortex with static stimuli, Neuroreport, 3 (1992) 369-372.
9 von der Malsburg, C. and Schneider, W. A neural cocktail-party processor, Biol. Cybern. 54 (1986) 29-40.
10 Wilson, M.A. and Bower, J.M. Computer simulation of oscillatory behavior in primary visual cortex, Neural Comput. 3 (1991) 498-509.
11 Young, M.P., Tanaka, K. and Yamane, S. On oscillating neuronal responses in the visual cortex of the monkey, J. Neurophysiol. 67 (1992) 1464-1474.