www.nazigassings.com

Diesel Gas Chambers
Ideal for Torture — Absurd for
Murder
Friedrich Paul Berg
1. Diesel Exhaust and Zyklon B
Most Nazi gassings were supposedly committed with Diesel
exhaust rather than cyanide or Zyklon B. Although this is contrary to
popular perceptions about the Holocaust story, Diesel exhaust has been
dominant, at least in terms of numbers of victims, in the claims of
holocaust scholars since the 1960's. The Diesel allegations did, however,
gain some public notoriety with the prosecution of American citizen John
Demjanjuk. Demjanjuk was accused of having murdered at least 875,000 Jews
with Diesel exhaust at the alleged extermination camp at Treblinka in
1942/43.
[1]
A nationally syndicated essay from one of America's
best-known newspaper columnists Patrick Buchanan raised the subject of
Diesel gassing to a fever pitch in the American press. Buchanan, a former
assistant to President Ronald Reagan, claimed that Diesel engines could
not kill at all.
[2]
His sweeping statement, which was far too broad,
brought him massive criticism but not for any valid technical reasons.
[3]
In 1992, a working draft paper authored by Walter Lüftl,
President of the Austrian Federal Chamber of Engineers, described mass
murder with Diesel exhaust as a "sheer impossibility."[4] Shortly
thereafter, he substantiated his view as to the relative harmlessness of
Diesel exhaust in an essay,[5] which was
publicly attacked as well.[6]
For readers familiar with auto emission issues, much of
what follows represents a kind of 'overkill' and rightly so. But in order
to put the Holocaust monster to its final, well-deserved rest – at least
its Diesel portion – one must be rigorous and even exhaustive. Since
Diesel gassings are not technically impossible, we must actually show how
it could have been done hypothetically, and then, just how thoroughly
unreasonable it is to believe Nazis or anyone would have ever used
the necessary technology.
In any event, all of the anti-Nazi gassing claims are
rubbish; Nazi gassings never happened!
2. Introduction
In any trial of even the most ordinary murder, one can
expect an abundance of information about the murder weapon. One would
expect the Allied and German post-war trials about murder as novel and as
bestially spectacular as the mass murder of millions of Jews in gas
chambers to provide the most extensive and precise documentation possible.
Although there is a vast literature based primarilyon those trials, as far
as the actual mechanics of the extermination process are concerned, all
one really finds is an occasional short and vague description.
Nearly sixty years have elapsed since the end of World
War Two. The Holocaust specialists have had more than enough time to
examine documents and alleged mass murder sites as well as testimony from
the most extensive trials in the entire history of the world. Throughout
this period they have been extremely active – but aside from a few bits
and pieces of so-called 'confessions' and 'eyewitness testimony,' they
have found next to nothing. The vast information gaps about the actual
mechanics of the alleged extermination process should arouse the gravest
suspicion.
Although the information gaps are bad for the
exterminationist position; what is even worse is that the few bits and
pieces of information are simply incredible. To characterize the alleged
mass murder methodology as 'hare-brained,' 'crackpot,' or 'weird' is to
understate the situation. If one looks at the claims critically, sooner or
later it becomes obvious that the people who repeat the Holocaust story in
one form or another simply have no idea as to what they are talking about.
The testimony of so-called 'eyewitnesses' is especially weird. The
statement by Kurt Gerstein, which for a long time was the evidence most
often used by Holocaust specialists, is the best example qualitatively.
The other 'statements' or 'confessions' are even worse.
The absurdities of the various alleged extermination
methods do not in and of themselves prove that the Holocaust did not
happen, but they should at least persuade reasonable people to ask for
some strong corroborating evidence. There are, for example, no autopsy
reports of gassing victims. The 'gas chambers' of Treblinka, Belzec and
Sobibor were all allegedly destroyed before the war ended. Those in
Auschwitz and Majdanek as well as those in the camps in the Reich proper
are ordinary rooms (mortuaries, shower rooms, delousing chambers) that
have been mislabeled 'gas chambers' in spite of their obvious design and
function – often to keep people alive.[7]
To concoct horrible but conveniently vague 'eyewitness'
accounts of mass murder is easy. To have such tales accepted about a
defeated enemy nation after a brutal war, during which the vast media
resources of the victors had already succeeded in portraying the enemy as
thoroughly depraved and wicked, is also easy. On the other hand, it is not
at all easy to explain how one could possibly commit mass murder with
Diesel exhaust. The exterminationists have never provided the necessary
explanation, not even in the great Israeli show trial of Ivan Demjanjuk,
where precisely such an explanation of the Diesel-murder-method should
have been demanded – at least by the defense.
3. The Exterminationist Position
Table 1 is from The Destruction of the European
Jews by Raul Hilberg and was published in 1961. The table summarizes
the views of practically all generally accepted 'consensus' writers on the
Holocaust story over the last 40 years. The camps listed are the only ones
still regarded as 'extermination' camps.
The fourth column from the left shows that in almost all
of the camps, the killing operation supposedly used carbon monoxide (CO).
In Auschwitz the killing operation supposedly used only hydrogen cyanide
(HCN). Of the five camps where carbon monoxide was supposedly used, the
vast majority of victims are said to have been killed in just three camps:
Treblinka, Belzec, and Sobibor. The carbon monoxide was supposedly
generated by Diesel engines. The number of Jews supposedly killed in
Kulmhof (Chelmno) or Lublin (Majdanek) are small compared to the numbers
for Treblinka, Belzec and Sobibor. The gas vans supposedly employed in
Russia also used Diesels.
On the basis of generally accepted numbers of victims,
nearly two-thirds of all the alleged Jewish victims of German gas
chambers were supposedly gassed with Diesel exhaust.
|
Table 1: Characteristics of the Death Camps
According to Raul Hilberg [8] |
|
Camp |
Location |
Jurisdiction |
Type of Killing Operation |
Number of Victims* |
|
Kulmhof
(Chelmno) |
Wartheland |
Higher SS and Police Leader (Koppe) |
gas vans (CO) |
150,000 |
|
Belzec |
Lublin district |
SS and Police Leader
(Globocnik) |
gas chambers (CO) |
600,000 |
|
Sobibor |
Lublin district |
SS and Police Leader
(Globocnik) |
gas chambers (CO) |
200,000-250,000[9] |
|
Lublin
(Majdanek) |
Lublin district |
WVHA (SS Economic-Administrative Main Office) |
gas chamber (CO, HCN)
shooting |
50,000-200,000[9]
|
|
Treblinka |
Warsaw
district |
SS and Police Leader |
gas chambers (CO) |
750,000
700,000[9]-1,200,000[10] |
|
Auschwitz |
Upper Silesia |
WVHA |
gas chambers (HCN) |
one million[11] |
|
* Updated figures were added here; cf. the
appropriate notes. |
For at least several months in 1939 and on into 1941, carbon monoxide had supposedly been used in Germany's euthanasia program to kill Germans who were feebleminded or incurably ill. The “experience” gained from this early and somewhat limited use of carbon monoxide from industrial gas cylinders under pressure was allegedly applied later in the spring of 1942 on a much grander scale to murder Jews, gypsies and others. A major change, however, was that diesel engine exhaust was used instead as the source of carbon monoxide–by some of the same people, such as Reichsamtleiter Viktor Brack and Kriminalkommissar Christian Wirth, to kill Jews in Treblinka, Belzec, and Sobibor in eastern Poland. According to Hilberg, it was Wirth who constructed the “carbon monoxide gas chambers” for the euthanasia program on the orders of Brack who was “actually in charge of the [euthanasia] operation.” Then in the spring of 1942, Brack ordered Wirth to Lublin where “Wirth and his crew immediately and under primitive conditions began to construct chambers into which they piped carbon monoxide from Diesel motors.”[12]
In 1978 and 1979, major American television network NBC
broadcast a four-day television miniseries entitled “Holocaust,” which was essentially a dramatization of the generally accepted Holocaust
story. There were several references to the use of Diesel engines for mass
murder. In one scene, Dr. Bruno Tesch, who in real life had actually been
a highly qualified chemist and was hanged after the war by the Allies,[13]
explains to Eric Dorf, a totally fictional SS officer administering the
extermination program, that one of the advantages of Zyklon B over carbon
monoxide is that Zyklon B "won't clog machinery – and there's no
apparatus to break down, as in carbon monoxide." In another scene,
Rudolf Höß, the commandant of Auschwitz, is about to start a Diesel engine
when Eric Dorf explains to him that he will not need the Diesel any longer
because he has ordered another substance, Zyklon B.
Reality is rather different from what was suggested in
the NBC miniseries and in some of the literature. The Zyklon B used in
Auschwitz consisted of granules made of gypsum and starch, which certainly
would have instantly clogged machinery and/or shower piping, although the
cyanide gas released from Zyklon-B granules would not clog anything.
Diesel exhaust does not clog machinery easily at all – unless the engine
is making smoke under an extremely heavy. This smoke contains some solid
matter which can, indeed, clog machinery (foul the cylinders), if there is
more smoke than can be blown out with the exhaust. Otherwise, no clogging
of cylinders occurs.
4. Kurt Gerstein
The statement of Kurt Gerstein remains a major
cornerstone of the Holocaust legend. Gerstein was an
Obersturmführer (First Lieutenant) in the SS and a mine surveyor by
profession with a graduate degree in engineering. When he surrendered to
the French, he supposedly gave them a prepared statement dated April 26,
1945. He had been elevated to the status of "righteous gentile" by
the Israelis and various Jewish writers for having at least tried to alert
the world to the Nazi extermination program. As H. Roques
pointed out,[14] six different versions of the Gerstein Statement
have been found to date and published by various researchers often in
grossly distorted and mutilated form.[15] Many
parts of Gerstein's statements range from the fantastically incredible to
the downright impossible. He allegedly committed suicide in a French
prison after having offered himself in vain as an informer to the French.
The trend in recent years has been to dissociate from him as 'witness for
the prosecution'. Nonetheless, his 'statements' are the only ones which
give at least a few technical details about the alleged Diesel gassings.
Raul Hilberg, for example, referred to his 'statement' many times without
actually quoting from it.[16]
The following text is an excerpt from the Gerstein
Statement as given in Harvest of Hate by Léon Poliakov. Aside from
a rather brazen 'error' on the part of Poliakov – namely the claim that
700 to 800 bodies were crowded into 93 square meters (1,000 sqf), instead
of only 25 square meters (269 sqf), which is the way the original document
actually reads – it is probably no worse than any of the other
translations which can be found:[15]
"SS men pushed the men into the chambers. 'Fill
it up', Wirth ordered; 700-800 people in 93 [sic; original claims
25] square meters. The doors closed. …
Then I understood the reason for the 'Heckenholt'
sign. Heckenholt was the driver of the Diesel, whose exhaust was to kill
these poor unfortunates.[17] SS Unterscharführer Heckenholt tried
to start the motor. It wouldn't start! Captain Wirth came up. You
could see he was afraid because I was there to see the disaster. Yes, I
saw everything and waited. My stopwatch clocked it all: 50 minutes, 70
minutes, and the Diesel still would not start! The men were waiting in the
gas chambers. You could hear them weeping 'as though in a
synagogue', said Professor Pfannenstiel, his eyes glued to the window in
the wooden door.[18] Captain Wirth, furious, struck with
his whip the Ukrainian who helped Heckenholt. The Diesel started up after
2 hours and 49 minutes, by my stopwatch. Twenty-five minutes passed. You
could see through the window that many were already dead, for an electric
light illuminated the interior of the room. All were dead after thirty-two
minutes!
Jewish workers on the other side opened the wooden doors.
They had been promised their lives in return for doing this horrible work,
plus a small percentage of the money and valuables collected. The men were
still standing, like columns of stone, with no room to fall or lean. Even
in death you could tell the families, all holding hands. It was difficult
to separate them while emptying the room for the next batch. The bodies
were tossed out, blue,[19] wet with sweat and urine, the legs
smeared with excrement and menstrual blood."
It is physically impossible to crowd 700 to 800 people
into a space of only 25 square meters, i.e., 28 to 32 people per
square meter.[20] According to Gerstein, it was not a peephole
through which Professor Pfannenstiel supposedly looked into the gas
chamber – it was a window in a wooden door, and not a gas-tight,
panic-proof steel door as one might expect. Supposedly, there were wooden
doors on two sides of at least one of the gas chambers. We are told that
the intended victims were still alive after almost three hours in the gas
chambers before the Diesel even started, so there must have been many air
leaks into the chambers or else the Jews would have been asphyxiated
without the aid of any Diesel.
There is no mention anywhere of the intended victims
trying to break out. Wooden doors with glass windows would hardly have
withstood a determined group effort to break through. Surely, Prof.
Pfannenstiel, with "his eyes glued to the window," would have
noticed if some people had been trying to smash the glass. But no, we are
told instead that the victims were calm enough and reflective enough to
form groups of family members, and hold hands, and even weep.
More than likely, Dr. W. Pfannenstiel, Professor of
Medicine at Marburg, had been sent to Belzec and other camps as medical
adviser to improve hygiene and health care in the camps. After the war, he
was repeatedly interrogated regarding his visit to Belzec with Gerstein.
He was charged in two cases but never convicted. In the court-room
statements, which are available to us, he never directly disputed
Gerstein's account, but in a private letter he described the Gerstein
Statement as "highly dubious rubbish in which 'fantasy' far outweighs
fact."[21] He also wrote that due to the persecution and
slander to which he was exposed, he did not wish to comment further on the
matter publicly. In other words, Pfannenstiel had clearly tried to avoid
further trouble for himself. The 'whole truth' would have been too much
for his prosecutors to bear.
According to the last sentence of the Gerstein text
quoted above, the bodies of the victims were "blue." Here we have a
major flaw as far as the death-from-carbon-monoxide theory is concerned
because victims of carbon monoxide poisoning are not blue at all. On the
contrary, victims of carbon monoxide poisoning are a distinctive 'cherry
red' or 'pink'.[22] This is clearly spelled out in most toxicology
handbooks and is probably well known to every doctor and to most, if not
all, emergency medical personnel. Carbon monoxide poisoning is actually
very common because of the automobile and accounts for more poison gas
injuries than all other gases combined.
The Gerstein statement, to its credit, does not claim
that carbon monoxide was the lethal ingredient in the Diesel exhaust. It
is the post-war exterminationists who insist that death was due to the
carbon monoxide in the Diesel exhaust. The recurrence of references to
"bluish" corpses in several other examples of so-called 'eyewitness
testimony' from West German trials merely demonstrates the 'copy-cat'
nature of much of that testimony. That such testimony has been accepted by
West German courts specializing in Holocaust-related cases and by the
Holocaust scholars, apparently without any serious challenge, merely
demonstrates the shoddiness of those trials and the pseudo-scholarship
which pervades the subject in general.
If the corpses had indeed appeared "blue," death
certainly would not have been due to carbon monoxide poisoning. A
bluish appearance could, however, have been an indication of death from
asphyxiation, i.e., from lack of oxygen..
According to Léon Poliakov, a French-Jewish historian who
has written at length in support of the Holocaust story,
"… there is little to add to this description
[the Gerstein Statement], which holds good for Treblinka and
Sobibor as well as for the Belzec camp. The latter installations were
constructed in almost the same way and also used the exhaust carbon
monoxide gases from Diesel motors as death agents."
According to Poliakov, more than a million and a half
people were killed with Diesel exhaust.[23]
5. Toxic Effects of Carbon Monoxide
To investigate the Diesel gas chamber claim, the two most
important questions are:
- How much carbon monoxide is actually needed to kill a human being in
half an hour?
- Does Diesel exhaust ever contain that much carbon monoxide?
Carbon monoxide poisoning has been thoroughly studied
since about 1920 when it was carefully examined to determine the
ventilation requirements of tunnels for motor vehicles, particularly for
the Holland Tunnel in New York City. Since the early 1940s, it has been
widely accepted on the basis of the research of Yandell Henderson and J.
S. Haldane that given a normal oxygen content of the air, an
average carbon monoxide concentration of "0.4% and above," as shown
on the last line of Table 2, is needed to kill people in "less"
than one hour of continuous exposure.[24]
Concentrations of 0.15%/vol. to 0.20%/vol. are "dangerous," which
means they might kill some people in one hour, especially if those people
have, for example, weak hearts. But, to commit mass murder in a gas
chamber one would need a concentration sufficient to kill not merely a
portion of any given group of people but rather, sufficient to kill
all. The prospect of 'survivors' of a gassing being 'regassed'
later on or disposed of in some other way is too ridiculous.
The vagueness introduced by Henderson's use of the term
"less" is unfortunate. It arises from the fact that although
Henderson and others could test for non-lethal effects in a laboratory
with a high degree of accuracy, the lethal effects could not be tested in
the same way. The lethal effects and the corresponding CO levels were
determined by careful extrapolation of carboxy-hemoglobin levels over time
from non-lethal tests on humans as well as from some lethal tests on
animals. Although the concentrations given for lethal effects are not as
precise as one might wish, they are still sufficiently accurate to support
some important conclusions about Diesel gas chambers.
According to the exterminationists, the gassing was
always done in about half an hour or less.[25]
To determine the carbon monoxide concentration needed to
kill in only half an hour instead of a full hour, one can use a widely
accepted rule of thumb known as "Henderson's Rule," which is:
%/vol. CO × exposure time = Constant for any given toxic
effect.
In other words, for any given toxic effect, the poisonous
concentration must be inversely proportional to the time of exposure. This
means that to kill in half an hour, one needs twice the concentration that
one would need to kill in a full hour. Applying this rule to the '0.4% and
above' needed to kill in "less than 1 hour," we get 0.8%/vol. as
the minimum concentration needed to kill in less than half an hour.[26]
|
Table 2: Toxic Effects of Carbon
Monoxide [27] |
|
Parts of carbon monoxide per million
parts of air |
Carbon monoxide
in
%/vol. |
Physiological
effects |
|
100 |
(0.01) |
Allowable concentration for an exposure of several
hours |
|
400 to 500 |
(0.04 – 0.05) |
Inhalation for up to 1 hour without appreciable
effect |
|
600 to 700 |
(0.06 – 0.07) |
Appreciable effect after exposure of 1
hour |
|
1,000 to 1,200 |
(0.10 – 0.12) |
Indisposition but no dangerous effects after
exposure of 1 hour |
|
1,500 to 2,000 |
(0.15 – 0.2) |
Dangerous concentrations for exposure of 1
hour |
|
4,000 and above |
(0.4 and above) |
Fatal in exposure of less than 1
hour |
Applying the same rule to the 0.15 to 0.20%/vol. range,
which is "dangerous" for one hour of exposure, we get 0.3%/vol. to
0.4%/vol. as the range of CO concentration, which is dangerous for half an
hour of exposure.
What all this means is that to have any kind of practical
gas chamber using carbon monoxide as the lethal agent, one needs an
average concentration of at least 0.4%/vol. carbon monoxide – but,
possibly as much as 0.8%/vol. We should keep '0.4% to 0.8%' in mind as
benchmark numbers to which we will refer shortly. Please note that these
data hold true only in the presence of a normal oxygen content of
the air!
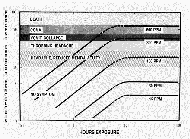 |
 |
|
Graph 1: Toxic effect of small amounts of carbon monoxide.[28] Top: original chart; left: additional
values extrapolated by the author.
(Click to enlarge)
|
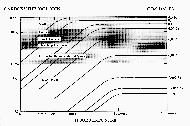
|
|
Graph 2:
100 cases of CO poisoning,
HbCO versus age. (Click to enlarge) |
If one were to reduce the oxygen content by half for
example – from the normal 21%/vol. to only 10.5%/vol. – any given
concentration of CO will be twice as toxic. Even a CO concentration of
only 0.2%/vol. would then suffice to kill in one hour. So, in order to
determine the actual effectiveness of a given concentration of CO, it is
necessary to see it in relation to the actual oxygen concentration
present. To properly use the values shown in our tables and graphs, one
must determine the CO content that would have the same effect with a
normal oxygen level as the actual CO content with reduced oxygen. This
concentration, which we shall call the "effective
CO-concentration," or c(COeff), is determined by
multiplying the actual CO-concentration, or (c(CO)), by the ratio of the
normal oxygen content (21%) to the actual oxygen content (x%):
|
c(COeff) = c(CO) × |
x% |
|
21% |
Another important consideration is always the average
concentration over the entire time of exposure, and not some quantity
of poison measured in pounds or cubic feet. In our current discussion,
this is a problem, since to determine the concentration one would like to
know the volume of the gas chamber which is not really possible here due
to the general lack of information. Neither is it possible to solve this
problem by determining an absolute quantity of poison instead of a
concentration value. The few data regarding gas chamber size, which we do
have for example from the Gerstein Statement, are so unbelievable that
there is no point in trying to work from them. But we do know that the
average CO concentration will always be less than the CO concentration
measured directly on the exhaust side of the Diesel engine.
Table 3 shows the Hb× CO levels
of carbon monoxide victims from the 1950s. In the literature of
toxicology, 60% Hb× CO is generally cited as the
fatal level (cf. Graph 1). According to Table 3, more than 1/4 of all
people would be dead at this concentration. Almost another 50% die at
levels up to 70% Hb× CO, and the last quarter
would die only when the concentration had increased to 80% Hb× CO (see also the scattergram Graph 2). So, to build
an effective CO execution gas chamber which, in keeping with eyewitness
testimony, kills everyone within half an hour – even young, healthy people
with good nerves – the chamber would have to reliably induce a level of
80% Hb× CO. An average CO content of 0.4% by
volume in the gas chamber air would be the absolute minimum required (cf.
Graph 1).
Graph 1 gives the symptoms from various low-level carbon
monoxide exposures as a function of duration of exposure. The highest CO
concentration discussed is 600 ppm (parts per million). 600 ppm is another
way of saying 0.06%/vol. The chart shows that after one hour of exposure
to an average concentration of 600 ppm of CO, one would experience a
headache, but not a throbbing one. Even after 100 hours of exposure, the
worst that one would experience would be unconsciousness, but not death.
However, after only half an hour of exposure to 600 ppm, no symptoms are
indicated at all – not even a mild headache. We should keep '0.06%' in
mind as another benchmark number to which we will refer later in this
chapter.
|
Table 3:
Hemoglobin-Carbon Monoxide Level of CO-Victims[29] |
|
|
Age of Victims
[years] |
|
Hb × CO
[%] |
18-30 |
30-40 |
40-50 |
50-60 |
60-70 |
70-80 |
80-90 |
Sum |
40-50
50-60
60-70
70-80 |
-
2
7
5 |
-
-
2
2 |
-
1
6
5 |
-
3
12
7 |
-
1
10
8 |
7
5
8
- |
4
5
-
- |
11
17
45
27 |
| Total: |
14 |
4 |
12 |
22 |
19 |
20 |
9 |
100 |
To obtain more reliable data about the effects of a
higher CO content in exhaust than those extrapolated here, one can consult
accident and suicide statistics. Accident or suicide victims who died from
carbon monoxide are frequently tested for the carboxy-hemoglobin (Hb× CO)[30] concentration in their blood.
What any would-be Nazi mass murderers
needed to achieve with their gas chambers is called by toxicologists the
"LD100," the lethal dose for killing 100%
of the victims. The concrete implications of this can be seen from the
statistical analysis of a study of 100 deaths from carbon monoxide
poisoning.
6. The Diesel Engine
6.1. Introduction
Although information as to engine type and size might be
considered essential in the investigation of any ordinary murder, such
details are just too much to expect when one is dealing with the Holocaust
hoax. Without more specific information, we must investigate the broader
and far more difficult question of whether or not any Diesel ever built
could possibly have done the abominable deed. The most frequent claim,
however, is that the engines were Diesels from Soviet tanks.[31]
If Gerstein had claimed that the carbon monoxide was
generated by gasoline engines, his story would be more credible. Gasoline
engines can indeed kill rather easily and with little or no warning
because their exhaust is almost odorless. Although Diesel engines look
like gasoline engines, at least to most people, they are actually quite
different. Any mining engineer or mine surveyor, such as Gerstein was,
should certainly have been able to easily distinguish between the two
types of engines. For one thing, the sound of Diesels is so distinctive
that almost anyone can with a little experience recognize them with his
eyes closed.
When Diesels are running, they actually warn us of their
presence: their exhaust smells terrible. In other words, every Diesel
engine comes with its own built-in 'warning ingredient.' The intensity of
the smell or stench has, no doubt, given rise to the thoroughly false
impression that Diesel exhaust must, therefore, be very harmful. The
simple-minded but false logic which guides Holocaust believers is that,
since gasoline engine exhaust can certainly kill, even though it has
little odor, Diesel exhaust, which has an intense odor, must be extremely
deadly. The fact is, however, that there is absolutely no relationship
between smell and toxicity since the most lethal ingredient by far is CO
which is totally odorless. Although Diesel exhaust is not totally
harmless, it is one of the least harmful pollutants anywhere except for
some possible long-term carcinogenic effects, which are totally irrelevant
for any gas chamber for mass murder.
Diesel emissions have until recently been well within the
air emission standards of the U.S. Environmental Protection Agency without
any modifications or accessories.[32]
However, concerns over cancer from Diesel exhaust have made the issue
quite complicated in recent years, but those concerns are only for
long-term effects. In any event, Diesels have always produced far less
than 1%/vol. carbon monoxide, which is still the CO standard for all
internal combustion engines. Gasoline engines have only met the same
standard after many years of intensive research and the addition of many
engine modifications and complex accessories including catalytic
converters.
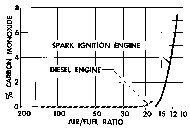 |
|
Graph 3: Comparison of carbon monoxide emissions from Diesel and
internal combustion engines.[33]
(Click to enlarge) |
Graph 3 compares carbon monoxide emissions from Diesel
and gasoline engines. The latter are also called spark ignition engines
because they use spark plugs. Clearly the logical choice between the two
types of engines as a source of carbon monoxide would always have been the
gasoline engine. From spark ignition or gasoline engines, one can easily
get 7%/vol. carbon monoxide – and with maladjustment of the carburetor, as
much as 12%/vol carbon monoxide – but from Diesel engines one can never
get so much as 12%/vol. with liquid fuels, except
during overloading.
Carbon monoxide emissions from internal combustion
engines are commonly plotted as functions of air/fuel ratio or fuel/air
ratio. Fuel/air ratio is merely the reciprocal of air/fuel ratio.[34] It has
generally been accepted by the automotive experts that the CO level of
Diesel exhaust is related chiefly to these ratios and not to other
factors, such as rpm.
An air/fuel ratio of 100:1, for example, means that for
every pound of fuel burned, 100 pounds of air are drawn into the engine.
However, only about 15 pounds of air can ever react in any way chemically
with each pound of fuel regardless of the air/fuel ratio or even the type
of engine. This means that at an air/fuel ratio of 100:1, there are always
about 85 pounds of air which do not react. These 85 pounds of excess air
are blown out of the engine without undergoing any chemical change at all.
As far as the excess air is concerned, the Diesel engine is nothing more
than an unusual kind of blower or compressor. In addition, there are no
adjustments that one can make on a Diesel to mistune the engine to change
the exhaust emission levels.[35]
Gasoline engines always operate with an air deficit. As a
direct result of this deficit, the combustion process in gasoline engines
can not possibly go to completion; a significant proportion of carbon
monoxide to carbon dioxide will always be formed.
Diesels by contrast always operate with excess air. At
idle, Diesels operate with air/fuel ratios as high as 200:1. At full load,
the air/fuel ratio is still only down to 18:1. Because of the abundance of
air, there is always far greater opportunity for the fuel to burn to
completion, thereby producing hardly any carbon monoxide. What little
carbon monoxide is produced in the cylinders of a Diesel is subsequently
diluted even further by the excess air.
Each cylinder of a Diesel either misfires or fires. If a
cylinder misfires, the fuel will simply be blown out as vapor and produce
no CO at all. When it does fire, the fuel will always burn to near
perfection because of the excess air which is always present.
Maladjustment, or faulty injection timing, or defective valves have no
significant effect on CO levels for the same reason: the excess air reacts
with nearly all of the remaining CO to form carbon dioxide.
As soon as one understands the true differences between
Diesel and gasoline engine combustion, the logical choice as a source of
carbon monoxide will always be the gasoline engine. The Diesel engine is
always an inherently ludicrous choice as a source of carbon
monoxide.
6.2. Divided Chamber Diesels
There are basically two types of Diesel engines: divided
combustion chamber and undivided combustion chamber. The divided chamber
category of Diesel engines is generally subdivided into pre-combustion
chamber designs and turbulent cell designs.
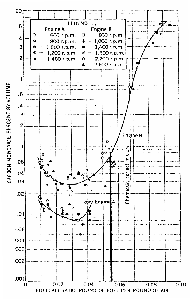
Graph 4 shows a pair of emission curves for Diesels with
divided combustion chambers (Engine A and B).[36] These
curves were the result of exceptionally careful tests made in the early
1940s in the United States by the U.S. Bureau of Mines (USBM) to determine
whether or not Diesel engines could operate underground without
endangering miners.[37] The conclusion of the USBM had been, at least until
the 1970's energy crisis, that Diesels could operate underground in
non-coal mines subject to USBM approval of the engines. Today, Diesels are
also widely used in U.S. coal mines. The earlier exclusion of Diesels from
US coal mines had nothing whatever to do with health and safety
considerations, but job security for coal miners and the political
persuasiveness and eloquence of John L. Lewis, the charismatic president
of the miners' union who had insisted: "no Diesels in UMWA
mines."[38]
The lower curve in Graph 4 is for a pre-combustion
chamber Diesel (Engine A). The upper curve is for a turbulent cell Diesel
(Engine B). The lowest fuel/air ratio always corresponds approximately to
idle and a 'no-load' condition. At idle, neither of these types of Diesels
could produce enough carbon monoxide to even give a headache after half an
hour of continuous exposure.
As one starts to impose loads on these engines, and in
effect increases the fuel/air ratios, the carbon monoxide levels actually
decrease at first. Only as one approaches full load, represented by the
solid heavy line in the figure, do the carbon monoxide levels rise
significantly to a maximum of 0.1%/vol. at a fuel/air ratio of 0.055. The
solid vertical line represents the safe maximum set by engine
manufacturers.
6.3. Undivided Chamber Diesels
 |
|
Graph 4: CO emissions from two different types
of Diesel engine: a precombustion chamber Diesel (A), a
turbulent cell Diesel engine (B).[37]
(Click to enlarge)
|
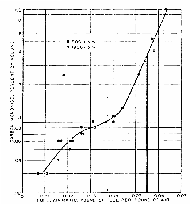 |
|
Graph 5: CO emissions from an undivided chamber
Diesel engine (C)[39]
(Click to enlarge) |
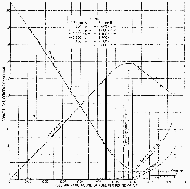 |
|
Graph 6: Composition of the exhaust from combustion
engines.[41] The heavy vertical line marking a fuel/air
ratio of 0.055 (air/fuel ratio 18:1) has been added by the author.
(Click to enlarge) |
The emission curve in Graph 5 (Engine C) shows that an
undivided chamber Diesel still produces only about 0.03%/vol. carbon
monoxide at idle, which is not enough to cause a headache in half an
hour.[39] However, as increasing loads are imposed on such an
engine, the carbon monoxide levels do eventually rise rather sharply. At
full load, represented by the heavy vertical line, the carbon monoxide
level is indeed at about 0.4%/vol. In other words, here we have a Diesel
which looks as if it could have been used to commit mass murder in half an
hour.
The problem for this engine, and for all Diesels, is that
to operate at full load continuously for long periods, such as half
an hour, one risks fouling and damage from accumulated solids inside the
cylinders. If one operates at lower and safer fuel/air ratios than 0.055
(air/fuel ratio 18:1), that is with lower loads, the carbon monoxide
emission levels drop very dramatically. For example, at 80% of full load,
which is generally regarded as the safe maximum for continuous operation
and which occurs at a fuel/air ratio of about 0.045 (air/fuel ratio @ 22:1), the carbon monoxide level is only 0.13%.
That the emission curves in Graphs 4 and 5 are indeed
typical of all Diesel engines over the last sixty years is attested to by
the fact that these particular curves have been referred to in countless
journals and books on Diesel emissions. In other words, there are no
better examples of Diesel emissions. To be sure, there are many other test
results in reputable automotive engineering journals such as the
Society of Automotive Engineers Transactions. But if one takes the
trouble to look through the SAE Transactions of the last sixty
years as well as through other journals, one will not find any examples of
worse carbon monoxide emissions than the curve in Graph 5 for engine C.
Our analysis of engine C represents the worst case that anyone is likely
to find anywhere, for any Diesel engine.[40]
6.4. Fuel/Air Ratios, Load and the Internal Speed
Governor
One might think that all one has to do to get a high
fuel/air ratio is to press the fuel pedal to the floor – without any
external load being coupled to the engine. What happens then, as the fuel
pedal is simply pressed 'to the metal,' is that the fuel/air ratio will
indeed go to the maximum that the fuel injection stop setting will allow
and, because of that, the engine speed will rapidly increase as well.
Within a few seconds, the engine speed will approach the maximum safe
engine speed set by the manufacturer. Long before that speed is reached,
however, an internal speed governor in the fuel injection pump assembly
will restrict the fuel supply – and quite severely – to protect the engine
by ensuring that the maximum safe engine speed or 'redline' speed is never
exceeded. The actual fuel/air ratio at 'high speed idle' will stabilize
after a few seconds, since there is no load, to nearly the exact same low
fuel /air ratio as at 'low speed idle.' At high speed idle, more fuel will
be consumed per second, but because more air is also being drawn into the
engine, the fuel/air ratio will remain nearly the same as at low speed
idle. In other words, pushing 'to the metal' without an external load will
not raise the fuel/air ratio, except initially.
To actually maintain a high fuel/air ratio for more than
just a few seconds, either of two methods, or a combination thereof, is
essential. One method involves coupling a load, such as a pump, fan, or
generator to the engine to hold the engine speed safely below the
'redline' speed. Another method is by 'choking,' which means restricting
the air supply to the engine.
As a practical matter, coupling an external load to an
engine in a typical truck or tank is far from easy. Nothing like it is
even remotely suggested in any of the anecdotes or documents anywhere in
the Holocaust literature. This method will be investigated more closely in
section 8.1.
Reducing the air intake, however, is quite easy, but
experiments have shown that this method still does not meet the necessary
requirements, see section 8.2.
7. Toxicology of Diesel Exhaust
7.1. Effect of Reduced Oxygen Content
Is it possible that the Jews died from reduced oxygen in
the Diesel exhaust? Such a theory would at least be consistent with the
claim that the corpses were "blue." A bluish coloring to certain
parts of a corpse is indeed a symptom of death from lack of oxygen. Normal
air contains 21%/vol. oxygen. In Graph 6 we see that the oxygen
concentration corresponding to idle in the exhaust of any Diesel engine
(divided or undivided chamber), shown at the right in the graph at an
air/fuel ratio of 100:1 (fuel/air ratio 0.01), is 18% which is just a few
percent less than one finds in normal air.[41] At
full load0 (fuel/air ratio 0.055), the oxygen concentration in the exhaust
of any Diesel engine is approximately 4%.
Probably the best discussion of the effects of
reduced oxygen levels, or asphyxia, is provided by Henderson and Haggard,
according to whom an oxygen content of less than 10%/vol. causes loss of
consciousness, and an oxygen content of less than 6%/vol. is fatal.[42]
According to Haldane and Priestley, "air containing less than 9.5 per
cent of oxygen would ordinarily cause disablement within half an
hour."[43] But disablement is still not death!
Clearly, there is no magic number below which death would
automatically occur, or above which life would necessarily continue.
However, for any gas chamber relying upon reduced oxygen as the killing
method, one would have to reduce the oxygen to below 9.5%/vol. and perhaps
even below 6%/vol.
From Graph 6 we see that to reduce the oxygen
concentration in the exhaust to just 9%, any Diesel would have to operate
at a fuel/air ratio of about 0.04, which corresponds to roughly
of full load. To reduce the
oxygen concentration to as low as 6%, a Diesel would have to operate at
close to full load. In other words, any Diesel gas chamber relying on the
reduction of oxygen as a killing method would have to operate at more than
¾ of full load.[44]
From the above it is evident that over most of their
operating ranges, Diesels discharge sufficient oxygen so that one can
literally inhale pure Diesel exhaust and survive. The smell will be
brutally unpleasant, but not harmful. From idle to at least
¾ of full load, Diesel exhaust
contains sufficient oxygen to sustain human life for at least half an
hour.
7.2. Combined Effects of Carbon Monoxide and
Reduced Oxygen
|
Table 4: Effective
CO-Content of Diesel Exhaust[45] |
| Load range |
Fuel/Air (Air/Fuel)
Ratio |
O2 Content
[%/vol.] |
COmaxContent
[%/vol.] |
FO2 |
COeff [%/vol.] at 21%/vol.
O2 |
|
Full load |
0.055 (18:1)
0.05 (20:1) |
4.0
6.0 |
0.400
0.220 |
5.25
3.50 |
2.100
0.770 |
|
Heavy load |
0.04 (25:1)
0.0333 (30:1) |
8.8
10.8 |
0.090
0.080 |
2.40
1.94 |
0.220
0.160 |
|
Partial load |
0.029 (35:1)
0.025 (40:1) |
12.0
13.5 |
0.075
0.070 |
1.75
1.55 |
0.130
0.110 |
|
Light load |
0.0167 (60:1) |
16.0 |
0.050 |
1.31 |
0.0667 |
|
Idle |
0.01 (100:1) |
18.0 |
0.060 |
1.17 |
0.070 |
Table 4 shows carbon monoxide levels for various load
ranges of the Diesel with the worst emission values, i.e.,
Engine C from Graph 5. When dividing the actual O2
content in the exhaust by the normal oxygen content in air (21%), one gets
a factor FO2. One can then multiply the
actual CO content by this factor to determine the toxicologically
effective CO content (see section 5). Table 4 shows us that the
desired, high effective CO content that guarantees the death of all the
victims within half an hour (0.4 to 0.8%) can only be attained near
full load.[46]
7.3. Carbon
Dioxide
If Jews were not killed with carbon monoxide or from a
lack of oxygen, could they have died instead from the effects of carbon
dioxide? Carbon dioxide is no more poisonous than ordinary water. Most
toxicology handbooks do not even mention it. When mentioned at all, it is
generally classified as a "non-toxic, simple asphyxiant." There
are, however, occasional accidental fatalities where carbon dioxide is
directly involved. Death in almost all such cases is caused by a lack of
oxygen. The lack of oxygen arises from the fact that the carbon dioxide is
much heavier than oxygen and will, especially in an enclosed space,
displace oxygen in the same way that water will displace air in the lungs
of a drowning man. The actual cause of death in either situation is not
the carbon dioxide or the water, but rather the lack of oxygen in the
blood (suffocation). One symptom of this kind of death is a bluish
appearance of the skin.
Carbon dioxide can be beneficial and therapeutic.[47] It is
commonly used in clinical medicine as a harmless stimulant for
respiration. For this purpose it is supplied under pressure in cylinders
(Carbogen) containing oxygen and 7%/vol. carbon dioxide.[48]
Normally, when a person exhales, the air leaving the lungs contains about
5.5%/vol. carbon dioxide.
Levels of 3%/vol. carbon dioxide are quite tolerable for
exposures lasting several days. For example, in the 1950s the U.S. Navy
experimented with gas mixtures containing 3%/vol. carbon dioxide and
15%/vol. oxygen (25% less oxygen than in normal air), for use in American
submarines with exposures lasting up to several weeks.[49]
For Diesel engines, the carbon dioxide level at or near
idle is only about 2%/vol. and gradually increases to about 12%/vol. at
full load as shown in Graph 6 (page 448 of Dissecting the Holocaust)). A carbon dioxide level of
12%/vol. may cause cardiac irregularity and may, therefore, be dangerous
for people with weak hearts.[50] In
contrast to Diesels, gasoline engines produce 12%/vol. already at idle. In
general, if enough oxygen is available, a carbon dioxide level even as
high as 12%/vol. is not likely to cause death. It is generally accepted
that only carbon dioxide concentrations greater than 20 to 30%/vol. are
dangerous.[51] However, when the carbon dioxide level is as high
as 12%/vol. in Diesel exhaust, the corresponding oxygen level is
dangerously low.
The principal danger to life from Diesel exhaust arises
not from any secondary components, but strictly from the combined effects
of CO and reduced oxygen.
7.4. Aldehydes, Sulfur Dioxide, Nitrous Oxides and
Hydrocarbons
Other pollutants in Diesel exhaust, besides carbon
monoxide, are primarily aldehydes (OCHR), sulfur dioxide (SO2),
nitrous oxides (NOx, max. 0.1%), and hydrocarbons
(CxHy). The smell or stench for which Diesel engines
are notorious is caused by trace amounts of certain hydrocarbons and
aldehydes which the most modern analytical instruments can barely
identify, let alone measure. The sensitivity of the human nose to these
compounds is, however, extremely high and out of all proportion to the
actual quantities present. Some of the hydrocarbons are considered
carcinogenic and thus represent a potential long-term hazard, but they are
irrelevant to our study.
The sulfur dioxide content of the exhaust, which can be
fairly high for sulfurous fuels, causes irritation of the respiratory
tract, but these irritations cannot become critical within the time frame
at issue here.
Nitrogen dioxide (NO2), if present in high
concentrations, can cause edema of the lungs after half an hour's
exposure. However, even the worst edema will not kill in half-an-hour, but
only after a delay of about 24 hours.[52]
One-time, brief exposure to lower concentrations of NO2 merely
irritate the lungs and mucous membranes, as do any sulfur oxides
potentially present, so that we need not consider them further. Nitrogen
monoxide (NO), on the other hand, has physiological effects similar to
CO.[53]
Unlike CO, however, its concentrations decrease with decreasing oxygen
concentrations in the combustion process, i.e., with higher load,
and do not attain any levels critical to health.[54]
Furthermore, NO converts rapidly to NO2,[55] so
that the NO concentration enhances the effects of the CO in the exhaust
only imperceptibly.
The peroxide (ozone) forming effects of nitrous oxides
near ground level as well as the carcinogenic components of Diesel exhaust
are the reason Diesel engines have recently also been subjected to special
emission guidelines. They supposedly pose a danger to human respiration.
This is why the studies conducted in Germany of health hazards posed by
Diesel exhaust were almost entirely confined to analyses of the
proportions of smoke solids and non-combusted hydrocarbons.[57]
7.5. Diesel Smoke
Diesels tend to smoke, especially under heavy load. This
is not due to any inherent inefficiency of Diesels. On the contrary,
Diesels are extremely efficient. The smoke is the result of the nature of
Diesel combustion and the heavier fuels which Diesels use compared to
gasoline engines.
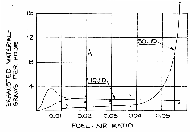 |
|
Graph 7: Liquids and
solids exhausted from engine per hr, and measured smoke.[56]
The heavy vertical line marking a fuel/air ratio of 0.055 (air/fuel
ratio 18:1) has been added by the author. (Click to
enlarge) |
The solid heavy lines in Graphs 4-7 represent the smoke
limit that manufacturers have found necessary to protect their engines
from excessive wear. As a practical matter, a Diesel cannot operate to the
right of the vertical lines in Graphs 4 and 5 (fuel/air ratio of 0.055 =
air/fuel ratio of 18:1) with liquid fuels because the internal
accumulations of smoke solids would destroy the engine within a short time
and would stall the engine.[58] Many manufacturers are more conservative and limit
their engines to fuel/air ratios below 0.050.
Diesel engines can operate safely at fuel/air ratios
above 0.055 (air/fuel ratios below 18:1) only if they are burning a clean,
gaseous fuel. This is the only way to avoid the buildup of solid material
within the cylinders. The data shown to the right of the vertical line
were only gathered because the researchers at the USBM chose to test their
engines for theoretical reasons with gaseous fuel far beyond the normal
(manufacturer recommended), full load settings of the respective
engines.[59] The data for clean, gaseous fuel is irrelevant to
our analysis because if the Germans had had a gaseous fuel for the Diesel
engines – for example, pure CO – they could have sent that gas directly to
the gas chamber. Using a Diesel engine as some kind of intermediate step
would have made no sense; it could only have made the gas far less toxic.
Since carbon monoxide is highly combustible and because of the excess air,
practically all of the carbon monoxide going into the Diesel would have
been consumed.
Diesel smoke contains a liquid phase and a solid phase.
The liquid phase generally gets blown out of the engine with the exhaust
and, therefore, can do no harm to an engine. But if enough solid material
is also produced, and rapidly enough, some of that material will
accumulate in the cylinders where, in just a few minutes, it can severely
damage piston rings and valves and even cause an engine to simply
self-destruct and stop. The amount of solids produced by Diesel engines
increases dramatically just above a fuel/air ratio of 0.055. For this
reason, manufacturers as a rule equip the fuel injection pumps with stops
so that the engines can only operate below 0.055, or even 0.050.
Operating any Diesel engine near the maximum load
recommended, regardless of the particular design or engine type, would
have produced significant amounts of smoke. Smoke is generally also
noticeable immediately after start-up, even at idle or under light load,
when the engine has not yet had time to reach its normal operating
temperature.
Pattle et al. found that an engine running at less
than half load and producing 0.22%/vol. CO also produces extremely
pungent, tear-inducing smoke which, if piped into a gas chamber, would
reduce visibility to a mere foot or so.[58]
It should surprise no one that there is no mention of
smoke from the Diesel – black, white, dense or otherwise – anywhere in the
Gerstein statement or in any of the postwar trial testimony. Can one
really believe Jews locked in gas chambers would have patiently withstood
such torture?
7.6. Noise, Vibration and Stench
The stench of Diesel exhaust is familiar to anyone who
has ever driven a car behind a truck or bus anywhere in the world. That
stench is, in effect, a powerful 'warning ingredient' to the presence of a
Diesel engine – at least until recent years when the addition of catalytic
converters and other equipment reduced the stench substantially.
Ironically, it was the removal of a warning ingredient in Zyklon B in 1944
which some holocaust believers have often cited as 'proof' of a fiendish
Nazi desire to deceive intended victims. With Diesel
exhaust, a technology to remove its warning ingredient simply did not
exist until many years later – and yet, the Nazis still
supposedly used Diesels for mass murder instead of, for example, gasoline
engines which have no such warning ingredient. In other words, the
arguments about 'warning ingredients' in connection with the 'Holocaust'
are at least as nonsensical as everything else.
Diesel engines are
also notorious for their intense noise and vibration. The noise and vibration are additional 'warning ingredients.' Because
of their higher compression ratios, lower rpm's, and the explosive type of
combustion – the amount of vibration Diesels produce is substantially
greater than that of any comparably-sized gasoline engines. The noise and
vibration are among the major reasons Diesels have not generally been
used in automobiles. They are too noisy for many people to bear.
If the 550 hp, V-12 cylinder Diesel from a typical Soviet
T-34 tank had been mounted on the floor of a small building and run for
half an hour at more than ¾ of
full load (at more than 375 hp), the noise and vibration would have been
at least as noteworthy and as wildly spectacular as the wailing of any
Jews – and yet, there is no mention of any such noise or vibration in the
Gerstein Statement, or in any of the post-war trial testimony.
click to continue with Part 2
www.nazigassings.com
NAZI Gassings Never Happened! Niemand wurde Vergast!