Brain Waves ("40Hz") Research
Introduction
Brain waves, or the "EEG", are electrical signals that can be recorded from the brain, either directly or through the scalp. The kind of brain wave recorded depends on the behavior of the animal, and is the visible evidence of the kind of neuronal (brain cell) processing necessary for that behavior.
We are working on fast brain waves, at about 40 cycles per second (Hz), which are known as gamma band. Gamma rhythms appear to be involved in higher mental activity, including perception and consciousness. It seems to be associated with consciousness, eg it disappears with general anesthesia.
Synchronous activity at about 40Hz appears to be involved in binding sensory inputs into the single, unitary object we perceive. This process is so efficient, we are hardly aware that it goes on at all. Recordings of neurons in visual cortex show that synchronization at about 40 Hz links parts of the cortex excited by the same object, and not those excited by different objects, implicating in gamma rhythms in binding. For instance the color, shape, movement and location of an object are processed in different parts of the visual cortex, and these features of an object need to be reunited into a single entity. This is known as the binding problem, and gamma rhythms are thought to provide a solution.
We have identified how the rhythm can be generated in small bits of cortex (see neuronal networks for gamma). Recently we have identified how gamma rhythms may be synchronized in separate parts of the brain (see long range synchronization of gamma).
Neuronal networks for gamma
Several mechanisms have been put forward for gamma oscillations. We have found that the networks of inhibitory neurons can produce 40 Hz or gamma rhythms without the need for the much larger population of excitatory neurons (the pyramidal cells), because the rhythms survive drugs which block excitatory synaptic transmission. The essential idea is that when you drive inhibitory neurons so that they want to fire faster than around 40 times a second, the inhibitory synapses between these neurons prevent them firing faster than every 25 ms or so (the precise time is determined by the size and duration of the inhibitory synaptic potential). Because each inhibitory neurons connects to many others, these connections also synchronize the population of inhibitory neurons.
Jefferys,J.G.R., Traub,R.D. and Whittington,M.A. (1996)
Neuronal networks for induced "40 Hz" rhythms.
Trends in Neurosciences 19, 202-208.
The rhythm depends on fast inhibitory synapses interconnecting the inhibitory neurons. It is driven by the steady activation of metabotropic glutamate receptors, and at least in principal by other slow excitation of inhibitory cells. Slow inhibitory synapses, using the GABA-B receptor, depress the rhythm, which links with parallel pharmacological studies of behavior in Prof Bowery's Group in Pharmacology.
Experiments and realistic computer simulations together have shown that this model works. Slowing the inhibitory potentials (IPSPs) between inhibitory neurons slows the gamma rhythm as predicted. The experiments are mainly on hippocampus. This is a part of the cortex with a relatively simple anatomy, which makes it convenient for most of the studies outlined here.
Traub,R.D., Whittington,M.A., Colling,S.B., Buzsaki,G. and Jefferys,J.G.R. (1996) Analysis of gamma rhythms in the rat hippocampus in vitro and in vivo. Journal of Physiology 493, 471-484.
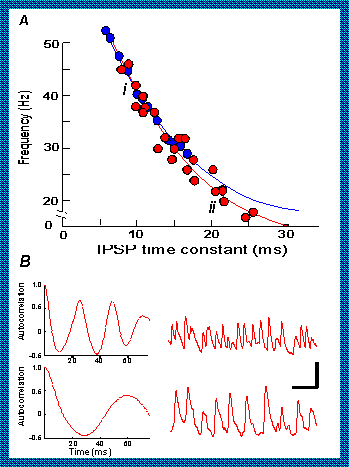
Simulations (blue circles) predicted, and experiments (red circles) confirmed that:-
1. Slowing the IPSP with barbiturates slows the oscillations.
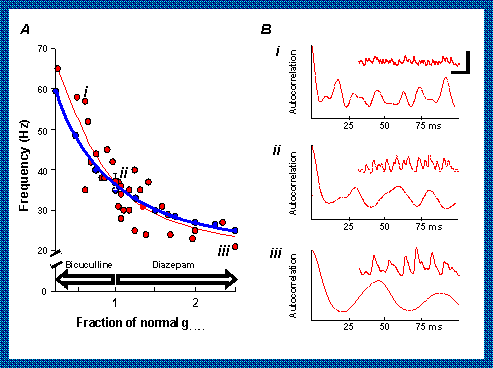
2. Making the IPSPs smaller with the drug bicuculline speeds up the rhythm and making them
bigger with diazepam slows it. The essential idea is that the smaller IPSPs take less time
to return the membrane to threshold for the next round of excitation. Part B shows data
for the parts of the graph labelled i, ii or iii.
Traub,R.D., Whittington,M.A., Colling,S.B., Buzsaki,G. and Jefferys,J.G.R. (1996) Analysis of gamma rhythms in the rat hippocampus in vitro and in vivo. Journal of Physiology 493, 471-484.
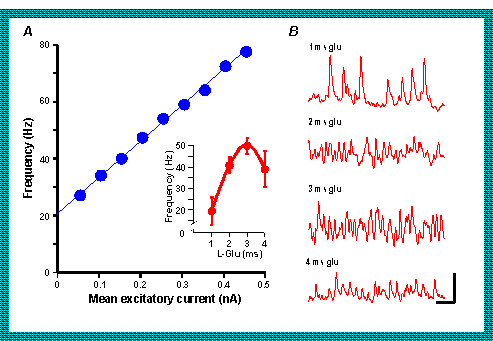
3. Making the tonic excitatory drive to the inhibitory neurons stronger speeds up the gamma rhythm
Traub,R.D., Whittington,M.A., Colling,S.B., Buzsaki,G. and Jefferys,J.G.R. (1996) Analysis of gamma rhythms in the rat hippocampus in vitro and in vivo. Journal of Physiology 493, 471-484.
4. The excitatory drive can change so that the precise frequency of the oscillation may alter during each response.
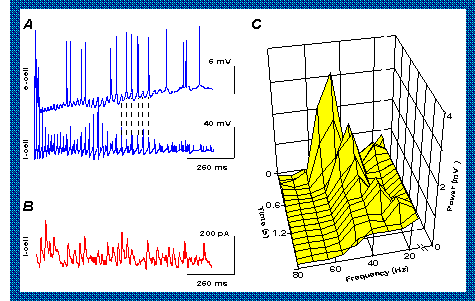
The latest step forward was to see how this works over long distances faster than axons (nerve fibres) joining regions. (See, press release)
Traub R.D., Whittington M.A., Stanford I.M. and Jefferys J.G.R. (1996) A mechanism for generation of long-range synchronous fast oscillations in the cortex. Nature 382, 621-624
"in phase": If both groups are synchronous, then the group 1 E cell spike takes (say) 5 ms to conduct (a) to group 2 where it can trigger a second spike in the I cells (and likewise from group 2 to 1 - not shown here).
"group 1 early": E cells are early in group 1 so the second spike of doublet it evokes in each group 2 I cell is earlier (b) and next cycle is shorter than it would otherwise be; the second spike of the group 2 doublet may fall into the spike refractory period and disappear, and thus shorten the next cycle even more. When group 1 is early, group 2 must be late, so the second spike group 2 E cells evoke in group 1 I cells is late (c), and the next cycle is longer. The end result is that the two groups tend to fall back in phase.
The computer simulations predicted that for this to work the system needed:
- each local network needs inhibitory connections (as before)
- local networks are connected together by connections to their inhibitory neurons from
BOTH the inhibitory neurons and
the excitatory neurons of their
neighbors.
Group members and their research
John Jefferys
Enrico Bracci
Martin Vreugdenhil
Stephen Hack
Collaborators
Roger Traub - Professor of Mathematical Neuroscience, Neuroscience Unit, Department of Physiology, University of Birmingham, formerly IBM Research Division, Yorktown Heights, NY
Miles Whittington - Imperial College School of Medicine at St Mary's, London
Recent Publications
- Whittington, M.A., Traub, R.D. and Jefferys, J.G.R. (1995). Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature 373, 612-615
- Traub R.D., Whittington M.A., Stanford I.M. and Jefferys J.G.R. (1996) A mechanism for generation of long-range synchronous fast oscillations in the cortex. Nature 382, 621-624
- Jefferys, J.G.R., Traub, R.D. and Whittington, M.A. (1996). Neuronal networks for induced "40 Hz" rhythms. Trends in Neurosciences 19, 202-208
- Traub, R.D., Whittington, M.A., Colling, S.B., Buzsáki, G. and Jefferys, J.G.R., Analysis of gamma rhythms in the rat hippocampus in vitro and in vivo, J.Physiol.(Lond.), 493 (1996) 471-484.
- Whittington, M.A., Jefferys, J.G.R. and Traub, R.D., Effects of intravenous anaesthetic agents on fast inhibitory oscillations in the rat hippocampus in vitro, Br.J.Pharmacol., 118 (1996) 1977-1986.
- Whittington, M.A., Traub, R.D., Faulkner, H.J., Stanford, I.M. and Jefferys, J.G.R., Recurrent EPSPs induced by synchronized fast cortical oscillations, Proc.Natl.Acad.Sci.USA, 94 (1997) 12198-12203.
- Traub, R.D., Whittington, M.A. and Jefferys, J.G.R., Gamma oscillation model predicts intensity coding by phase rather than frequency, Neural Comput., 9 (1997) 1251-1264.
- Traub, R.D., Jefferys, J.G.R. and Whittington, M.A., Simulation of gamma rhythms in networks of interneurons and pyramidal cells, J.Comput.Neurosci., 4 (1997) 141-150.
- Whittington, M.A., Stanford, I.M., Colling, S.B., Jefferys, J.G.R. and Traub, R.D., Spatiotemporal patterns of gamma-frequency oscillations tetanically induced in the rat hippocampal slice, J.Physiol.(Lond.), 502 (1997) 591-607.
- Colling, S.B., Stanford, I.M., Traub, R.D. and Jefferys, J.G.R., Limbic gamma rhythms. I. Phase-locked oscillations in hippocampal CA1 and subiculum, J.Neurophysiol., (1998) (In Press)
- Stanford, I.M., Traub, R.D. and Jefferys, J.G.R., Limbic gamma rhythms. II. Synaptic and
intrinsic mechanisms underlying spike doublets in oscillating subicular neurons,
J.Neurophysiol., (1998) (In Press)