
Color pictures 1-4: © Karl Philipp
(© Karl Philipp)
(© Karl Philipp)
(© C. Mattogno[316])
(© C. Mattogno[316])
(© Carlo Mattogno[318])
Fig. 66:
Zyklon B disinfestation chamber in Stutthof camp, east side, exterior. (© Carlo Mattogno[318])6. Formation and Stability of Iron Blue
Hundreds of thousands of people are claimed to have been killed in the alleged Auschwitz 'gas chambers' by hydrogen cyanide in the form of the product Zyklon B®. The question which now arises is the following: could this poisonous gas leave chemical traces, which could perhaps be detected in these alleged chemical slaughterhouses?
If hydrogen cyanide (HCN), the reactive compound in Zyklon B, were only bound to the walls by adsorption (adhesion),[313] there would not be any detectable residues today anymore, due to the volatility of hydrogen cyanide (boiling point: 25.7°C); all the hydrogen cyanide involved would long since have evaporated.
But if one assumes that the hydrogen cyanide, during fumigation, would combine with certain materials in the masonry to create other, considerably more stable compounds, then one might anticipate the possible existence of chemical residues even today.
The reaction products of interest to us in this respect are the salts of hydrogen cyanide, called cyanides,[314] in particular, the iron cyanide group, formed by a compound of iron and cyanide. Iron occurs universally in nature. It is iron which gives brick its red color, sand its ochre color, and clay its color ranging from yellowish to reddish-brown. More precisely, we are speaking of iron oxide, popularly known as 'rust'. Basically, all walls consist of at least 1% rust, as a result of sand, gravel, clay, and cement, of which the wall is constructed.
The iron cyanides have long been known for their extraordinary stability, one of them having achieved particular fame as one of the most commonly used blue pigments during the last three centuries: Iron Blue, also often referred to as Prussian Blue.[315]
6.2. Instances of Damages to Buildings
Chapter 1.3. contained a discussion of an instance of damage to a church which occurred in 1976 in Bavaria, Germany. In the many hundreds of thousands of fumigations which have been carried out since 1920, there cannot, as a rule, have been any complications, otherwise the procedure would have been very rapidly abandoned. The case in question was, therefore, an exception. But what exactly was it that made this church an exception?

|
Color Image 1: Inside of the ruins of morgue 1 (‘gas chamber’) of crematorium II. The arrow points to the photograph taking location (see chapter 8.3.3.). Note: Not the slightest trace of blue discoloration can be found on the walls. Color pictures 1-4: © Karl Philipp |
|
Interior northwest room in the Zyklon B disinfestation wing of BW 5a in the Birkenau camp. (© Karl Philipp) |
Exterior southwest wall of the Zyklon B disinfestation wing of BW 5b in the Birkenau camp. (© Karl Philipp) |
|
Zyklon B disinfestation installation, chamber III, of barrack 41 in Majdanek camp. (© C. Mattogno[316]) |
Zyklon B disinfestation installation, east wall of chamber III of barrack 41 in Majdanek camp. (© C. Mattogno[316]) |
| Large Zyklon B disinfestation chamber, ceiling, barrack 41 in Majdanek camp. (© C. Mattogno[316]) | Zyklon B disinfestation installation, chambers II and III (exterior wall), of barrack 41 in Majdanek camp. (© Carlo Mattogno[317]) |
|
Zyklon B disinfestation chamber in Stutthof camp, interior view taken from the south door. (© Carlo Mattogno[318]) |
Fig. 66: Zyklon B disinfestation chamber in Stutthof camp, east side, exterior. (© Carlo Mattogno[318]) |
| Color Image 4 (inset): Exterior of a wall of the same part of BW 5a. Cyanide penetrated the wall and caused blue stains. | Color Image 11: Blue discoloration of the east exterior wall of the Zyklon B disinfestation installation in the Stutthof camp, section enlargement of Color Image 10/Fig. 66. © C. Mattogno. |
Different scenery. 1939-1945. In the camps of the Third Reich, hundreds of thousands of people-Jews, political prisoners, criminals, 'anti-socials,' and prisoners of war-were crammed together. To stem the raging epidemics, attempts were made, not always with great success, to kill the carriers of disease, particularly head lice. This was done in particular with hydrogen cyanide, Zyklon B. This was sometimes done in chambers professionally designed for such purposes, sometimes ordinary rooms were equipped for such purposes in an auxiliary manner, and provisionally used for disinfestation. Many of the camps in the Third Reich were leveled at the end of the war or afterwards; in other camps the existing buildings were torn down and the buildings materials used for the reconstruction of the ruined cities. A few buildings, however, remain intact today. The interiors of these buildings look as in Fig. 59-66 (see also the color picture section in the middle of this book).
From the remarks of a Polish research team having conducted investigations on behalf of the Auschwitz Museum, we also know that the disinfestation chamber in the Auschwitz main camp is colored a spotty blue.[56],[57] To my knowledge, only the Zyklon B disinfestation chambers of Dachau camp (DEGESCH circulation chambers) exhibit no blue pigmentation, because the walls were professionally coated with a paint impermeable to gas and water.
It seems therefore that a blue pigmentation of masonry is no exception, but rather a rule, particularly where unprotected masonry is repeatedly exposed to hydrogen cyanide over long periods. The large-scale, long-term use of hydrogen cyanide for vermin control in disinfestation chambers only began, in practice, with the onset of the Second World War. And with the dissolution of the National Socialist prisoner camps, the confiscation of the corporation having manufactured and marketed Zyklon B (the I.G. Farbenindustrie AG), and the invention of DDT at the end of World War II, this large-scale use of hydrogen cyanide ended just as abruptly. No one cared about any 'instances of building damage' having occurred in the former National Socialist disinfestation chambers in this period. The question never arose in the literature... until Frederick A. Leuchter came along.
The following is an attempt to demonstrate the manner in which these blue pigments, referred to as Iron Blue, came to be formed in the masonry during fumigation with HCN, and the conditions favorable to their formation.
There have been many publications on this chemical compound in the last five decades, which were perused and will be summarized in the following in relation to our topic. In so doing, attention was directed at:
When writing the initial versions of this expert report intended to be presented at German courts of law, I was extremely anxious not to make any errors, because I knew that the topic was extremely controversial. As a consequence, I over-examined several chemical aspects involved, some of which can be understood only by chemical experts. Others aspects are not really necessary for an understanding of the core issue. In order to have a complete English version of my expert report, I nevertheless decided to include all the material I accumulated over the years. Those sections, however, which are considered of marginal interest or of interest to experts only, I have given headlines always starting with "Excursus". For some readers it might be advisable to skip these chapters. They will most likely not miss anything.[319]
But first a short description of the starting substance, hydrogen cyanide.
6.3. Properties of Hydrogen Cyanide, HCN
Hydrogen cyanide, a colorless liquid, is similar, in many of its physical properties, to water.[320] This similarity also explains the limitless solubility of HCN in water and its strong tendency towards absorption (dissolution) in water. The equilibrium concentration[321] of hydrogen cyanide in water is investigated in more detail in chapter 6.5.3.
The opinion is often expressed that, because gaseous hydrogen cyanide is approximately 5% lighter than air, it must separate from air and rise. Hydrogen cyanide gas is, however, only slightly lighter than air and does not separate, because of the thermal movement of every gas particle. To clarify this, reference must be made to the principal components of air: The main component of air, nitrogen, 78% by volume, is 8% heavier than hydrogen cyanide gas. If a separation took place between hydrogen cyanide gas and nitrogen, it would all the more occur between the two main components of air, since oxygen (21% of air by volume) is 15% heavier than nitrogen. This would have as a result that all the oxygen of the earth's atmosphere would settle in the lower fifth of the atmosphere, as a consequence of which the entire surface of the earth would get oxidized, i.e., burn. This obviously does not happen. Thus, a spontaneous separation of hydrogen cyanide gas would never take place in air.
|
Table 3: Physical Properties of HCN[322] |
|
|
Molecular weight |
27.026 g mol-1 |
|
Boiling point (1 atm) |
25.7°C |
|
Melting point |
-13.24°C |
|
Specific density of the gas at 31°C (air = 1) |
0.947 |
|
Explosion limits in air |
6-41 Vol.%[323] |
The 5% lesser density of pure hydrogen cyanide gas compared to air (this corresponds to a density difference of 35°C warm air as compared to 20°C warm air) can however very well lead to a density convection, when pure gaseous hydrogen cyanide is released in a location with the same temperature as the ambient air. The gas would then rise slowly, but gradually mix with the ambient air. But to conclude from this that hydrogen cyanide vapors always rise, would be an incorrect conclusion. At 15°C, for example, on physicochemical grounds, no concentrations higher than 65% of hydrogen cyanide can occur in air (see Graph 1); the density of such a mixture lies only approximately 3% below that of air. Furthermore, a great deal of energy is withdrawn from the ambient air by the evaporating hydrogen cyanide. Consequently, the ambient temperature sinks until exactly as much energy is transported to the liquid (adsorbed) HCN as needed for the decelerated evaporation at the corresponding lower temperature. It is therefore theoretically possible for hydrogen cyanide vapors containing little HCN, but which are cold, to be denser, that is, heavier than the ambient air.
|
|
|
Graph 1: Vapor pressure of hydrogen cyanide in percentage of air pressure as a function of temperature. |
Graph 1 shows the equilibrium percentage of hydrogen cyanide in air as a function of temperature. Even at 0°C, the percentage still lies at approximately 36% by volume. Condensation of HCN on surrounding objects would occur only if the percentage rose over the equilibrium percentage (the so-called dew point). Since in all cases here under consideration, a maximum concentration of 10% HCN in air would only be reached for a short period of time close to the source of HCN (the Zyklon B carrier), no condensation of HCN on walls can be expected. An exception is, however, the so-called capillary condensation, which can occur in finely porous materials such as cement mortar.[324]
Hydrogen cyanide forms explosive mixtures with air in the range of 6 to 41% by volume. With strong initial ignition, its explosive effects can be compared with nitro-glycerin.[325] In the applications under discussion here, a proportion of 6% by volume and more can be reached in the immediate vicinity of the source, which suffices for local blow ups at the most. Hence, only inappropriately high concentrations can lead to explosive mixtures, as shown by a corresponding accident in 1947.[16] With correct application quantities and concentrations, the technical literature indicates that there is practically no danger of explosion.[326]
The stoichiometric composition of an ideal Iron Blue crystal is:
Fe4[Fe(CN)6]3
It is characteristic that the iron in this compound is present in two different oxidation states: Fe2+ (here in square brackets) and Fe3+ (here on the outer left). The interaction between these two different iron ions also gives rise to the blue color of this compound (Charge-Transfer-Complex). The actual composition can be quite variable, depending on the stoichiometry on formation and the presence of impurities, in which case the color varies between dark blue and greenish-blue tones.
It was with support of the Mösbauer spectroscopy[327] that a long-lasting argument could be decided:[328],[329] Turnbull's Blue, Fe3[Fe(CN)6]2, is actually the same as Berlin Blue, Fe4[Fe(CN)6]3, even if the summation formulas suggest they are different. As a matter of fact, the summation formula of Berlin Blue is closest to the reality: In the ideal Iron Blue crystal, 16 molecules of coordination water are included:
Fe4[Fe(CN)6]3 · x H2O (x=14 to 16)
Today it is known that the 'soluble' Iron Blue which frequently is referred to in older literature, is mainly a substance with the composition MeFeIII[FeII(CN6)] · x H2O, where Me is the counter ion to the opposite cyanoferrate, [Fe(CN)6]3-/4-, mostly potassium (K+) or ammonium (NH4+).
According to Buser,[329] 'soluble' Iron Blue is formed mainly during quick formation and precipitation of the pigments, leading to the inclusion of large amounts of water and potassium or ammonium ions in the extremely voluminous precipitate. The resulting crystal is therefore very faulty and more appropriately called a polymer.[330] By filtration, drying and intensive grinding, however, this very inhomogeneous, polluted Iron Blue can be transformed into a pigment which is only hardly colloidal dispersible.[331] This 'soluble' Iron Blue is not soluble in the original sense of the word, but can more easily be colloidally dispersed than the 'insoluble' Iron Blue, which is very important for its application as a pigment.[332],[333]
However, these colloids are very instable and precipitate easily when salts are added.[334] According to Buser,[329] even in presence of high concentrations of potassium ions, almost pure 'insoluble' Iron Blue can be obtained, if the formation process is proceeding slowly enough. In case of deeper interest about the structure one might consult the following literature.[329],[335]
We are only concerned, in this connection, with how Iron Blue arises from hydrogen cyanide and iron compounds in building materials. In building materials, the iron is generally present in trivalent form (Fe3+), in the form of 'rust'.
For the formation of Iron Blue, therefore, a part of this iron must be reduced to bivalent form (Fe2+). The subsequent combination of these different iron ions with CN- to Iron Blue occurs spontaneously and completely.[336] The most probable mechanism[337] is one in which the cyanide ion itself acts as a reducing agent. The starting point in so doing is an Fe3+ ion, largely surrounded (complexed) by CN- ions: [Fe(CN)4-6](1-3)-. A slightly alkaline environment is favorable to the final reduction of the iron(III)-ion to iron(II).[338]
The pigment formation in the case under consideration here is then organized in 5 steps:
The velocity of formation of the pigment can be influenced by various factors, which will be considered:
The formation of cyanide through absorption and subsequent dissociation of hydrogen cyanide in water is the necessary precondition for a reaction with iron compounds, since the hydrogen cyanide itself exhibits only a low reactivity. All reactions listed in chapter 6.5.1. under a)-e) occur almost exclusively in water. Water furthermore ensures that the reaction partners-all salts capable of being dissolved in water-come together in the first place. Finally, the moisture contained in the building material also acts as a hydrogen cyanide trap, since hydrogen cyanide dissolves eagerly in water. A relatively high water content in the masonry will therefore considerably increase the speed of reaction.
The reason for the low reactivity of HCN compared to the free cyanide ion is because HCN is less nulceophilic than the free ion.[341] Aside from the dissociation of hydrogen cyanide in water, the process of chemisorption[313] on solid surfaces deserves being mentioned, where the hydrogen cyanide releases its proton (H+) to an alkaline oxide and is itself attached to a metal ion.
Absorption and dissociation of the superbly soluble hydrogen cyanide (see chapter 6.5.4.) is clearly superior to chemisorption. Furthermore, the aqueous solution (as solvent) is indispensable for the complex formation and redox reactions of the cyanide with Fe3+. Additionally, the aqueous medium makes the reacting agents mobile, which do not always form at the same location. And finally, the moisture contained in the solid material works as a trap for hydrogen cyanide, because it intensely binds the hydrogen cyanide. Or the other way around: the drier a solid material is, the easier hydrogen cyanide, which was ad-/absorbed before, will be released back into the environment. Therefore, a relatively high water content of the solid material will accelerate the reaction.
Experiments with reactions of hydrogen cyanide (some 4 g per m³ in air, 15°C, 75% rel. humidity) with mixtures of Fe(OH)2-Fe(OH)3 attached to wet paper strips showed that a blue discoloration occurred after 30 min at a pH value[342] of 2 to 3, since at such low values almost no hydrogen cyanide dissociates to the reactive cyanide (see chapter 6.5.5.). At pH values of 7 to 9, a visible blue discoloration occurred after a few minutes of inserting the sample. At higher pH values, this time span grew again, because the initially absorbed hydrogen cyanide had to lower the pH value first, before it could form the pigment (see chapter 6.6.1., pH Sensitivity).
These experiments show clearly that undissociated, gaseous HCN or HCN dissolved as gas shows no reactivity. An addition of small amounts of KCN to an aqueous sulfuric acid solution of Fe2+/Fe3+, however, results in the immediate precipitation of the pigment. The cyanide obviously reacts faster with the iron salts than it is protonated by sulfuric acid, i.e., converted into hydrogen cyanide.
6.5.3. Reactivity of Trivalent Iron
The solubility of trivalent iron diminishes rapidly with increasing alkalinity (rising pH value). Even in a pH neutral environment, almost all iron is bound as rust.[343] The reaction between iron compounds and cyanide resulting in the formation of the intermediate product iron(III)-cyanide, [Fe(CN)6]3-, is therefore largely a reaction on the solid-liquid interface, that is, between the iron adhering to the solid body and the cyanide ion in solution. This reaction occurs considerably more slowly than the same reaction in an aqueous solution. The fastest possible reaction requires a large surface area on the solid-fluid phase boundary, that is, a large, interior, microscopically rough surface and a fine, highly porous solid body, since in such cases, a lot of the iron compounds lie on the surface and are therefore less solidly bound and can quickly combine with cyanide.
In an increasingly alkaline environment, only decreasingly small amounts of 'rust' can slowly be converted into iron(II)-cyanide, but cannot react with iron(III)-ions to form Iron Blue.
Even in an alkaline environment, it must be expected that rust, in the presence of perceptible cyanide concentrations, will be quite slowly transformed into iron(III)-cyanide and finally into iron(II)-cyanide.[344] The last step required for the formation of Iron Blue, however, the combination of iron(II)-cyanide with iron(III), will not occur due to the lack of dissolved iron(III)-ions. In a strongly alkaline environment, an increasing concentration of iron(II)-cyanide, which is chemically stable, can slowly accumulate. It remains in a stand-by position, waiting for the pH value to drop.
Iron salts generally tend to incorporate water, and Iron Blue is no exception to this. A higher water content in the solid body results in increased water accumulation in rust, too. The rust expands, so to speak, and thus becomes more reactive towards competing ligands[345] like cyanide. Freshly precipitated, extremely moist and non-homogenous iron hydroxide precipitations possess extreme reactivity, and together with hydrogen cyanide, as shown in chapter 6.5.2.2., they form the pigment in visible quantities in minutes.
For the formation of colloidally dispersible Iron Blue, the quick formation in aqueous solution with high concentrations of the agents is required (see chapter 6.4.2.), since this leads to heterogeneous crystallites (tiny crystals) with many inclusions (ions, solvent molecules) and a high degree of disorder. These crystallites have only a small tendency to coagulate.
The slow interface reaction on the phase-boundary layer liquid-solid with quite low concentrations of the reacting agents will suppress the formation of colloidally dispersible Iron Blue. The process described here, occurring in walls exposed to hydrogen cyanide, strongly resembles the formation of monocrystals as described by Buser,[329] since in this case also, one reagent (Fe2+) had to be formed through slow reduction by excess cyanide. Thus, except from the inhomogeneous material, the conditions here under consideration are suitable for a slow crystal growth of insoluble Iron Blue without large amounts of inclusions and formation of crystal defects.
The environmental temperature influences larger magnitudes in quite a different manner:
A: Graph 2 shows the maximum solubility of HCN in water at various temperatures with a hydrogen cyanide content of 1 mol% in air,[346] which corresponds to approximately 13 g hydrogen cyanide per m3 air.[347] It increases, as with any gas, with decreasing temperature and lies between 0.065 mol per l at 30°C and 0.2 mol per l at 0°C.
|
|
| Saturation concentration of hydrogen cyanide in water as a function of temperature at 1 mol% HCN in the air (partial pressure[348] of p(HCN)=0.01, e.g., 10 mbar HCN at 1,000 mbar total pressure). |
These high concentrations prove the extreme solubility of hydrogen cyanide in water.[320] It decreases by approximately half every 20°C. It is therefore approximately 10,000 times more soluble in water than oxygen (O2) and approximately 250 times more soluble than carbon dioxide (CO2). The latter is not without importance, since the opinion is sometimes expressed in the literature that the carbon dioxide content of air could have an influence on the amount of hydrogen cyanide soluble in water.[57] But since hydrogen cyanide is considerably more soluble in water than CO2, and since CO2, furthermore, is hardly converted into carbonic acid in water at all, this influence may be disregarded.[349]
B: The moisture content of masonry is very strongly dependent on the relative humidity of air and the temperature. Since the tendency of water to evaporate (water vapor pressure) increases with rising temperature, and since, as a rule, the relative humidity of the air decreases, and since both lead to a drop in the water content, any increase in the temperature has a cumulative effect. Drops in water content by a power of ten at temperature increases of 10°C have been proven in the temperature ranges of 10-30°C under consideration (see chapter 6.7.).
C: Only an acceleration in the slowest of the five steps described in chapter 6.5.1. can be responsible for a change in the velocity of the entire reaction. In neutral or alkaline medium, this is the displacement of the oxygen or OH--ion in rust by the cyanide ion (point c). Although the iron(III)-cyanide [Fe(CN)6]3- itself is stable in a moderately alkaline medium[350]-that is, the iron(III)-cyanide is more stable than the rust-the displacement of the OH--ions in rust is inhibited by the cyanide, since the rust is not dissolved in water. An increase in temperature by 20°C usually doubles the velocity of reaction, if the other parameters remain unchanged.
This is, however, not so in extreme cases, since, as shown above, the velocity of reaction is very strongly negatively influenced by the massively decreased water content at higher temperatures (see above): decreased mobility of the reaction partner, decreased reactivity of iron, increased evaporation of ad-/absorbed hydrogen cyanide etc. (see chapters 6.5.2. and 6.5.3.). A strong reduction in pigment formation must therefore be expected at increased temperatures.
A decisively higher water content of the solid material and the considerably better absorption and solubility properties of hydrogen cyanide in water are the reasons for the tendency of solid materials to accumulate more cyanides with lower temperatures. An increase in the reactivity of iron oxide (rust) in the solid body with relation to hydrogen cyanide with a higher water content of the solid material at lower temperatures must be anticipated, as well as with a general increase in the reactivity of all agents. A cooler, and thus moister, solid material is therefore better suited to the formation of Iron Blue than a warm, dry body.[351]
There are two more steps in the observed reaction which could, theoretically, have an influence on the reaction under consideration:
A: The adsorption of hydrogen cyanide on solid surfaces decreases with rising temperature, according to Langmuire (see Graph 3).[352]
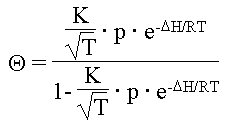 |
(1) |
|
Q |
= Degree of adsorption |
D H |
= adsorption enthalpy (negative) |
|
K |
= variable |
R |
= universal gas constant |
|
T |
= temperature |
e |
= Euler's number (2.71828...) |
|
p |
= gas pressure |
|
|
| Degree of coverage of the surface of a solid material with an adsorbed gas as a function of temperature (schematic) |
The intensity of the decrease of the equilibrium degree of adsorption (coverage) with rising temperature as well as the point of approximate saturation, however, are unknown for the problem at hand. But since, as discussed before, all reactions under consideration require aqueous solutions anyway, adsorptions on solid, i.e., dry surfaces are of no importance to our investigation.
|
|
|
Graph 4: Degree of disassociation of hydrogen cyanide as a function of the pH value at room temperature. |
B: According to the literature, the dissociation behavior of acids as a function of temperature is not unanimous.[353] Although there is a tendency of increasing protolysis[354] with rising temperature, this tendency turns upside down at higher temperatures for some acids, others show generally falling values. Since the changes are generally in the range of low percentages only, and because speed of protolysis is generally very high, hence never a restricting factor, this can be neglected here.
The pH value (acidity) influences the formation in various ways. In chapter 6.5.1., reference has already been made to the higher reduction power of cyanide and iron(III)-cyanide in alkaline environment. The pH value also influences the reactivity of iron compounds in the solid body (chapter 6.5.3.).
|
|
|
Graph 5: Cyanide equilibrium concentration in water as a function of the temperature and pH value at 1mol-% HCN in the air. |
As remarked above, dissolved hydrogen cyanide hardly exhibits reactivity. The formation of cyanide ions by absorption and dissociation of hydrogen cyanide only starts in sufficient degree at neutral pH values and above, see Graph 4.[355] The data leading to Graph 4, together with the data that enabled us to plot Graph 2 (saturation concentration of HCN as a function of temperature), leads to a graph revealing the relationship between temperature, pH value (acid content), and CN- saturation concentration, see Graph 5 (at a concentration of 1 mol% HCN in air, which is approximately 1% by weight, the usual disinfestation concentration).[356] At neutral pH values, equilibrium concentrations of CN- are within the range of 3×10-4 to 1×10-3 mol per liter, depending on the temperature. An increase in the pH value by one point results in a ten-fold increase in the cyanide equilibrium concentration. The actual cyanide concentration in masonry is determined by the velocity of absorption of the gas, adsorption effects within the solid material, and possible reactions of the cyanide.
The result of all these factors is that slightly alkaline pH values are favorable to the formation of the pigment.
The individual parameters and their influence on the formation of Iron Blue are summarized in the following table:
Table 4: Formation of Iron Blue | |
|
Parameter |
Effect |
|
Water content |
Increase in water content results in the following: increased absorption of hydrogen cyanide; long-term retention of ad-/absorbed hydrogen cyanide; increased mobility of reaction partners; increased reactivity of iron oxide; water is the basic precondition for disassociation and redox reactions; generally positive influence with increasing water content. The water content is dependent above all upon the temperature. |
|
Reactivity of the iron |
Factor determining reaction velocity, apart from the type of material and pH value (see below), positively influenced by increasing water content. |
|
Temperature |
Increased ad-/absorption of hydrogen cyanide as well as-under otherwise identical conditions-decreased velocity of individual reactions with falling temperature; strong increase on water content, and therefore a strongly positive net influence upon all other factors with a falling temperature. |
|
pH value |
Increased iron reactivity with falling pH, as well as a massive reduction in cyanide accumulation and redox reactivity of iron(III)-cyanide; compromise between iron reactivity and cyanide formation/Fe3+ reduction: A weakly alkaline pH value is favorable to absorption of hydrogen cyanide and accumulation of cyanide as well as for the reduction in iron(III)-cyanide, which determines the velocity of the reaction. Although more strongly alkaline media can accumulate iron(II)-cyanide over longer periods of time, no Iron Blue can form under such circumstances. |
Iron Blue is an extremely acid-resistant, but base-decomposing pigment.[357] Hydrogen cyanide is only released by warm, diluted sulfuric acid, while hydrochloric acid, by contrast, has no effect.[358] In a clearly alkaline environment, i.e., in the presence of high concentrations of OH- ions, these displace the cyanide ion from the iron(III)-ion. Fe(OH)3 is then precipitated ('rust sludge'), and the Iron Blue is destroyed.[359]
The literature contains authenticated cases of studies with iron at pH values of 9 and 10, in which it is still stable.[360] The pH range around 10 to 11 can be considered the critical limit for the stability of Iron Blue. Based on the alkaline behavior of fresh mortar and concrete (in this regard, see also chapter 6.7.2), Iron Blue is only used to paint these surfaces to a limited extent.[361]
Iron Blue is considered one of the least soluble cyanide compounds, which is the precondition for its widely-varied application as a pigment.[362] The literature flatly refers to Iron Blue as "insoluble".[363]
Concrete, reliable values on the solubility of Iron Blue are not recorded in the scientific literature. Based on comparative calculations between the known solubility of Fe(OH)3 on the one hand, and the limit value of the pH stability of Iron Blue on the other hand (pH 10), the approximate solubility of Iron Blue in water can, however, be calculated (see chapter 6.6.2.2.). It amounts to approximately 10-24 g Iron Blue per liter of water, this means that 0.000000000000000000000001 g Iron Blue dissolve in 1,000 g of water.
In addition to a compound's solubility in water, its condition (crudely or finely crystalline, superficially adherent or adsorbed by capillary effects) as well as, in particular, the condition and quantity of the water supplied are decisive in determining the actual velocity of dissolution of a substance. Iron Blue formed in masonry will be present in a finely crystalline form and adsorbed by capillary effects, in which case the former favors dissolution, while the latter is extremely detrimental to dissolution. Water almost or entirely saturated with iron(III)-ions is no longer capable of dissolving further iron. Furthermore, water permeation through finely porous solid material like masonry is extremely low even at high water tables; the iron saturation concentration is quickly attained, which, in addition, as remarked above, is generated by the slightly more soluble iron oxides of the solid body rather than by the Iron Blue having once arisen. It is furthermore very well known that mortar and concrete permeated with paints practically cannot be rendered colorless.[364] It cannot, therefore, be anticipated that the Iron Blue content once having arisen in walls can be perceptibly reduced by dissolution in water. Water running down the exterior surfaces is considerably more aggressive, exerting, in particular, an erosive effect, i.e., damaging the masonry as such.
Tananaev et al.[365] examined the solubility of metal hexacyanoferrate(II) and discovered a solubility product[366] of 3 · 10-41 (pKS =40.5) for the solubility product of Iron Blue, without mentioning the unit used.
Assuming they used the summation formula of Fe4[Fe(CN)6]3 (unit being mol7 l-7), one attains a solubility of 0.5 mg per liter water. Thus, it would be 14 times less soluble than the nearly insoluble calcium carbonate (CaCO3, 7.1 mg per liter water, KS = 4.95 · 10-10 mol2l-2).[367] Later publications support these findings,[336] although attention must be paid to deviations in the stoichiometry (composition) of Iron Blue with impurities, leading to an increased solubility.
Tananaev et al. precipitated the complex metal cyanoferrate from an appropriate metal salt solution with Li4[Fe(CN)6], probably acquiring a high rate of inclusions (lithium, water) as well. Thus, in spite of the four hour-long accumulation of the precipitation, the filtrate would certainly still have contained colloidally dispersed Iron Blue. Since they finally determined the amount of free Fe3+ in the filtrate by precipitating it with ammonia as Fe(OH)3, they will undoubtedly also have precipitated the Fe3+ of the colloidally dispersed Iron Blue, as ammonia raises the pH value so much that Iron Blue is no longer stable (see chapter 6.6.1.).
Therefore, they did not determine the solubility of Iron Blue, but the measure of stability of the dispersion of fresh precipitations of the pigment.
The solubility product of Pb2[Fe(CN)6] given by Krleza et al.,[336] which they used as a reference to determine the solubility products, is much lower than the one used by Tananaev et al.. If applied to Tananaev's calculations, this produces a solubility of Iron Blue of only 0.05 mg per liter. Krleza et al., however, find similar results for the solubility of most of the metal cyanides analyzed, including Iron Blue. Since conventional methods of analysis, such as gravimetry and titration, tend to be unreliable when facing minute traces, one must but wonder about these similar results.
However, one can escape this dilemma by thoughtful reasoning.
It is safe to say that Iron Blue is stable at a pH value of 7, i.e., in a neutral aqueous medium, so we take this as a minimum value. As mentioned earlier, a pH value of about 10 can be considered the upper limit of stability for Iron Blue, so we take this as maximum value for the following calculations. At pH=7, and even more so at pH=10, the free iron concentration is extremely low, since Fe(OH)3 is nearly insoluble (see Table 5).
At pH 7 and 10, respectively, a saturated Fe(OH)3 solution has the following free Fe3+ concentration:
|
c(Fe3+) = |
KL(Fe(OH)3) |
(2) |
|
c3(OH-) |
|
pH=7: = |
2.67×10-39 mol4l-4 |
= 2.67×10-18 mol l-1 |
(3) |
|
10-21 mol3l-3 |
|
pH=10: = |
2.67×10-39 mol4l-4 |
= 2.67×10-27 mol l-1 |
(4) |
|
10-12 mol3l-3 |
Should the free Fe3+ concentration surpass this value due to a better solubility of Iron Blue, then Fe3+ would precipitate as hydroxide and would be increasingly removed from the pigment, thereby destroying it in the end. Since this does not happen at pH=7 at all, and pH=10 can be considered the point where it just starts to happen, the concentration of the Fe3+ ion in a saturated Iron Blue solution must lie well below 10-18 mol/liter, i.e., in the area of 10-27 mol/liter. Thus, the solubility of Iron Blue must also have a value around 10-27 mol per liter (actually: 1/4 of the free Fe3+ concentration, KS less than 4.1 · 10-187 mol7 l-7, pKS larger than 186.6) which, at a mol mass of 1,110 g mol-1 ((Fe4[Fe(CN)6]3 · 14 H2O) would correlate to 10-24 g.
|
|
|
Graph 6: Free Fe³+ concentration as a function of pH value and the resulting minimal pKS value of Iron Blue, depending on its stability at the corresponding pH value. pKS value acc. to Tananaev et al.: 40.5; according to reflections made here: greater than 123, smaller than 186. |
With this, the complex iron pigment does indeed deserve to be called insoluble, as only one part of dissolved Iron Blue can statistically be found in 100,000,000,000,000,000,000,000,000,000 parts of water (1029). The actual solubility would therefore be less by a factor of 1020 as determined by Tananaev et al., which would come pretty close to values calculated for other so-called 'insoluble' compounds, like mercury sulfide (HgS). However, one must consider that the chemistry of Fe3+ in aqueous solutions doesn't justify the terms 'dissolved' or 'precipitated', since a multitude of complexes do exist in the broad pH-spectrum, partly as polymer hydroxo-aquo-complexes (compare chapter 6.5.3.).
Graph 6 shows the correlation between the pH value of the free Fe3+-concentration in a hypothetical saturated solution of Iron Blue and the respectively resulting minimal pKS values possible for Iron Blue, which it must possess, should stability prevail at the given pH-reading. Tananaev's pKS value given, it is obvious that the pigment would remain stable only up to pH 3. Accordingly, it would dissociate itself by its eigen pH value of 4 (see chapter 6.6.1., note [358]), which is formed in its own dispersion. Thus the magnitude of error in the results of Tananaev et al. and Krleza et al. is apparent.
These reflections show that iron, bound as hydroxides or oxides in solid materials, tends to dissolve in a neutral medium more readily than Iron Blue, since its equilibrium concentration must be higher than that of Iron Blue.
6.6.3. Excursus: Competing Ligands
|
Table 5: Dissociation constants and solubility products |
||
|
Compound |
Constant |
Source |
|
KS(Fe4[Fe(CN)6]3) |
4.1 · 10-187 mol7 l-7 |
|
|
KD(6)([Fe(CN)6]4-) |
10-24 mol l-1 |
|
|
KD(6)([Fe(CN)6]3-) |
10-31 mol l-1 |
|
|
KS(Fe(OH)2) |
4.79×10-17 mol3 l-3 |
|
|
KS(Fe(OH)3) |
2.67×10-39 mol4 l-4 |
|
|
KS(FeCO3) |
3.13×10-11 mol2 l-2 |
|
As shown, OH--ions may, due to the low solubility of Fe(OH)3, noticeably precipitate the Fe3+ of Iron Blue in pH media above 9 to 10. The residual hexacyanoferrate(II), on the other hand, would only decompose in strongly alkaline media, because Fe(OH)2 is simply more soluble (compare Table 5).[369]
Tartrate[370] has, in contrast to oxalate, hardly any effects so that Fe3+ can be quantitatively removed from sour wine with [Fe(CN)6]4-, a usual procedure to remove iron ions from wine.[371] Concentrated alkali carbonate solutions will precipitate the Fe2+ of Iron Blue as FeCO3, so that they destroy the entire pigment by precipitating Fe3+ as Fe(OH)3 (due to alkalinity) and the hexacyanoferrate(II) salt [Fe(CN)6]4-.[372] Calcium carbonate solutions, however, would not be sufficient due to their marginal saturation solubility. Besides that, Kohn examined the supportive effect of most of the organic ligands to disperse Iron Blue.[373]
Thus, apart from OH- (alkaline medium), there are no other ligands to be considered competing in the formation or dissolution of Iron Blue in the cases here under consideration.
Iron Blue itself is generally considered a light-resistant pigment, which is only slowly decomposed by the effects of UV radiation.[374] Thus, there are even patents utilizing Iron Blue as a UV-absorbing pigment, which is only meaningful with sufficient resistance to UV radiation.[375] Since the walls of interest to us here are protected from UV radiation and because UV radiation can only exert a superficial effect on the walls, while the Iron Blue would form and remain within the walls, a possible process of decomposition by UV radiation can have no influence upon our investigation.
Certain wave lengths of ultraviolet radiation may set free CN- from hexacyanoferrate(II) and -(III), the preliminary stages of Iron Blue. As far as hexacyanoferrate(III) is concerned, this leads to the formation of Iron Blue.[337] As far as hexacyanoferrate(II) is concerned, quantum efficiencies[376] of 0.1 to 0.4 are reported for wave lengths of 365 nm.[377]
Lately, it has been discussed whether complex cyanides can be removed from industrial waste waters by ultraviolet radiation. The unbound cyanide will be oxidized and destroyed by hydroxyl radicals originating from the parallelly occurring photolysis of water.[378] However, results are not unequivocal.[379]
As for Iron Blue, one knows of the bleaching effect under strong, perpetual sun radiation and the ensuing re-darkening during the night.[380] Here also, the liberation of CN- is responsible, which reduces parts of the Fe3+ ions to Fe2+ ions. The latter process, however, will reverse during the night under the influence of oxygen and moisture. The Iron Blue concentration will eventually be reduced by the loss of the released CN-, either by evaporation of hydrogen cyanide, by washing out as CN-, or by oxidation through Fe3+/atmospheric oxygen or from hydroxyl radicals from the natural photolysis of water. The latter process is minute and can therefore be omitted. At any rate, most of the cyanide released by photolysis will again be complex bound to iron.
The best long-term test available to us consists of disinfestation buildings BW 5a and 5b in Birkenau, which have defied the wind and weather of the strongly corrosive climate in the industrial region of Upper Silesia for over 50 years, and which are still colored blue, both inside and out, exhibiting a high cyanide content. These findings are also supported by two other long-term tests.
The color durability of Iron Blue, in addition to other pigments, was tested during an environmental resistance test lasting 21 years in the industrial district of Slough, west of London.[381] In so doing, pieces of aluminium sheet metal were alternatively dipped in an iron(III)-cyanide and then in an iron(III)-salt solution,[382] by which the resulting pigment was adsorbed on the aluminium sheet metal. The test sheets were then exposed to the environment on the roof of a building in a vertical 45° angle facing south-west.
During the 21 years lasting test, in which eight Iron Blue samples were tested among other pigments, the Iron Blue, in particular, followed by iron ochre (Fe2O3, rust), exhibited only minimal alterations after this period of time. One sample of Iron Blue and iron ochre was removed only after 10 to 11 years in each case.[383] All other samples still exhibited an intense blue color. Half of the seven remaining Iron Blue samples received the value 4 out of a maximum of 5 points for the best retention of quality, on the grey scale used there in the determination of color changes. Only minor alterations were detected.
The exhibits were therefore exposed to the environmental conditions of a strongly industrialized area, with full effects of precipitation, direct sunshine, and wind erosion for more than 21 years. Under intense summer sunshine and in the absence of wind, the temperature of the dark-blue colored aluminium metal sheets rose steeply (Iron Blue is only stable up to approximately 140°C.[384]). Snow, frost, hail, storms, and the finest, driving acid drizzle had obviously just as little an effect on the pigment as the UV radiation of direct sunlight. What is remarkable is that in determining the degree of destruction of the pigment no unexposed samples were used since these had been lost over the 21-year period; rather, places on the surface of the exhibits which had been relatively well protected from direct environmental influences by the frames and by rubber rings on the screw joints were used as control samples. These exhibited almost no alterations.
In comparison to the environmental conditions which are of interest here, this long-term test involved considerably more severe conditions, since in this case, the externally formed Iron Blue was only superficially adsorbed upon the aluminium sheets. The pigment nevertheless resisted extremely well.
Another event proves the extraordinary long-term stability of Iron Blue. For many decades at the end of the 19th and the early decades of the 20th century, Iron Blue was a by-product in the generation of city gas, because the hydrogen cyanide contained in coke gas had to be eliminated for security reasons by washing it with iron hydroxide prior to introduction into the city gas network. Iron Blue is the end product of this washing process. City gas works frequently disposed of this product by distributing some of it over their factory terrain with the intend to kill weeds-in vain, though, since Iron Blue has no effect as an herbicide. Today, the grounds of former German city gas works still contain high quantities of Iron Blue, many decades after the works were put out of operation. It was neither decomposed, nor dissolved or washed away by rain water, since it is insoluble. In particular, terrain with a high Iron Blue content is not considered polluted, since it is physiologically unobjectionable due to its stability.[385]
In summary, it may be stated that Iron Blue having formed in the interior of a wall as a component of the wall itself, possesses a longevity comparable to the iron oxide from which it has formed. This means simply that Iron Blue possesses a degree of stability which is comparable to that of the masonry itself: the Iron Blue will remain contained in the wall for as long as the wall itself remains in existence.[386]
Once perceptible quantities of cyanide have accumulated within a wall, therefore, and once conditions permit the conversion of the cyanide into Iron Blue, no perceptible reduction in the Iron Blue content can be anticipated, even after fifty years or more.
A typical example of the manner in which the media deal with these facts is a press report issued by the German Press Agency (Deutsche Presseagentur, dpa) on March 29, 1994, and which was then published in many German newspapers and even broadcast on radio. The report flatly claimed that, according to unnamed experts:
"Cyanide compounds decompose very quickly. In the ground, this occurs even after six to eight weeks; in masonry, these compounds could only be preserved under 'absolute conditions of conservation including complete exclusion of air and bacteria."[387]
Inquiries with the dpa press office in Stuttgart which published the report revealed that the writer responsible for the report, Albert Meinecke, had simply invented this expert opinion.[388] This obvious lie continues to be further disseminated, even by German government agencies such as, for example, the Bavarian Ministry of the Interior.[389]
6.7. Influence of Various Building Materials
Bricks are well-known to acquire their hardness and stability during their baking process. This causes an intensive binding of the components in bricks (sintering). One result of this is that the reactivity of the iron oxide occurring in bricks (2 to 4%) is strongly reduced, so that a perceptible inclination to form iron cyanide is hardly to be anticipated. The immediate surface of bricks slightly attacked by atmospheric influences (weathering) nevertheless constitutes an exception to this rule, so that the superficially adherent iron oxide is available for conversion into Iron Blue.
The chemical composition of bricks varies massively due to the different sorts of mar and loam used as initial material. The content of clay (included in this are 20 to 60% Kaolinite, consisting roughly of 47% SiO2, 40% Al2O3, 13% H2O) may lie between 20 and 70%, the rest being carbonate, finest sand and iron oxides.[390] According to my own analyses, the latter content may vary between 2 and 4%.
The porosity values of bricks lie between 20 and 30 vol.%,[391] according to other sources up to 50%.[392] According to my own mercury penetration tests, the pore size of bricks lies heavily concentrated around 1 µm.[393]
Due to the decreased specific surface (0.5 to 1 m² per g, BET,[394] own tests), the reactivity of the iron oxide is strongly reduced. However, partly dissolved iron at brick surfaces immediately exposed to weathering can be set free for reactions in bigger amounts.
The normal free, i.e., not chemically bound water content of bricks in dry rooms (20°C) is in the area of one volume percent, but it can rise up to 4% at a relative humidity of over 90%.[395]
6.7.2. Cement Mortar and Concrete
The rust content (Fe2O3) of Portland cement, of particular interest to us here, the cement most frequently used for concrete and cement mortars, is usually between 1 and 5%.[396] The sand added to the mortar can also exhibit a high iron content (up to 4%). As mentioned in chapter 6.5.3., a large surface area at the solid-liquid phase limit (iron oxide-cyanide solution) is favorable to the formation of Iron Blue. This is extraordinarily large in cement and concrete mortars (microscopic interior surfaces of approximately 200 m2 per gram).[397]
Fresh concrete and cement mortars-which are identical from a chemical point of view-are relatively strongly alkaline (pH approximately 12.5). It later falls, however, due to the binding of carbon dioxide from the air. Depending on the special chemistry of the cement mortar, this process proceeds very slowly in the depth of the material. According to the composition of the cement mortar, this may last from a few months to many decades, until the pH value of such a mortar or concrete becomes neutral, even in the deepest layers.[396]-[398] This chemical behavior explains the entire secret of the stability of reinforced concrete, which prevents the embedded steel from rusting further in the environment within the concrete, which remains alkaline for lengthy periods of time.[399]
The water content of concrete and cement mortars depends on the temperature and relative humidity of the air and fluctuates between 1% and less at 20°C and 60% relative humidity up to 10% in air saturated with humidity.[395] In case of permanently high humidity, penetrating wetness from outside, a huge part of the pore system can be filled with water.[400]
Poorly insulated rooms built underground always have cool and humid walls due to their great exchange surface area with the ground: partly because of their absorption of humidity from the ground, and partly because of the condensation of humidity in the air on the cool walls when the temperature falls below the dew point. The water content of these walls therefore lies around 10%, i.e., a factor of approximately 10 or more above that of dry walls of heated rooms built above ground.
|
Table 6: Composition of Portland cement[402] |
|
|
Al2O3 : 5 to 10 % |
K2O: 0.2 to 0.6 % |
|
SiO2 : 20 % |
Na2O: 0.5 to 3 % |
|
CaO : 60 % |
Fe2O3 : < 5 % |
The chemical composition of Portland cement, the most frequently used cement for concrete and water mortar, can be seen in Table 6.
The specific surface of the cement powder is in the order of 3,000 cm2 per g. Concrete and cement mortar get their stability by hydration of the cement compounds calcium oxide CaO (burnt lime), silicium dioxide SiO2 (Quartz), iron and aluminium oxide Fe2O3/Al2O3 to mixed, microfibrous calcium alumosilicate-hydrates with a chemically bound water content of some 25 mass %.[401] It then has a specific surface of up to 200 m2 per g when measured with water adsorption, which is an extremely high value. Other methods (e.g. BET-measuring with nitrogen) yield a value of only 1/3 of this or less.[397] The porosity of mortar and concrete heavily depends on the amount of water added during preparation and lies at a minimum of 27% according to the literature,[400] in which case the volume of the microcapillary pores between the silicate fibers is included as well, which cannot be determined with mercury penetration measurings.
|
|
|
Graph 7: Accumulated pore volume distribution of concrete, according to "Forschungs- und Materialprüfungsanstalt, Abteilung 1: Baustoffe" (Research and Material Testing Agency, Department 1: Building Materials), Stuttgart, and of wall mortar, own analysis. In each case determined by Hg penetration. |
Aside from the absolute porosity, the pore size distribution is decisive for the reactivity towards gases. If the main pore volume is formed by micropores, then the gas diffusion into the material is more inhibited than if the main pore volume is formed by larger pores. Graph 7 shows the accumulated pore volume distribution of concrete and one wall mortar (exact composition unknown, since taken from an old wall, but according to its brittle consistency probably a lime mortar).
Having a similar total pore volume like the wall mortar (here only 14% due to the test method), the concrete's largest portion of pore volume lies between a pore radius of 0.01 and 0.1 µm, whereas the wall mortar's largest portion lies between 0.1 und 10 µm. Hence, if compared with the wall mortar, the gas diffusion into the concrete will be disadvantaged. In general, the average pore size of cement building materials changes to larger values when increasing the content of sand and lime.
Fresh concrete is relatively strongly alkaline, caused by the high content of calcium hydroxide, which, however, gets bound as calcium alumosilicates rather quickly. However, depending on the type of cement, a certain amount of it is released as time goes by. The pH value of non-carbonated concrete is around 12.5. It later falls, however, due to the binding of carbon dioxide from the air.
The speed of carbonation into the depth of the concrete depends strongly on the consistency and porosity of the material and follows a square root relation:[403]
|
D = c ·t1/2 |
(5) |
|
|
d = depth of carbonation |
In water tight concretes, it takes many years for the limit of carbonation to advance only a few centimeters due to the inhibition of diffusion in this highly compact material.
In the area of carbonation, the pH value decreases to roughly 7, the equilibrium value of saturated calcium carbonate solutions. But if the wall is wet, this results in a proton exchange and therefore no sharp pH border is formed. If a large portion of the air pores (size in the order of a tenth of a millimeter) flooded with water poor in carbon dioxide, the carbonation advances more slowly, because compared to the gaseous phase, diffusion in aqueous phases is much slower, by some orders of magnitude. In case of waters rich in carbon dioxide, however, this can accelerate the carbonation.
The iron content of lime mortars is based, in particular, on the admixed sand (up to 4 % Fe2O3). Lime mortar is manufactured using only burnt lime (CaO), sand, and water and acquires its solidity through the binding of slaked lime (Ca(OH)2) with atmospheric carbon dioxide to lime (CaCO3). This procedure takes only days or weeks (depending on the thickness of the particular layers), due to the cruder porous system which facilitates the diffusion of gas. For fresh lime mortar, an extreme high water content can be damaging, as the carbon dioxide necessary for the binding process can no longer penetrate into the wall.
The final ph value of this material lies within the neutral range. Since this medium no longer provides sufficient protection for steel reinforcement rods and offers only slight environmental resistance, it is usually used for the plastering of interior walls and for interior brick walls only, in the latter case often mixed with cement.[400] The specific surface of lime mortar lies considerably beneath that of cement mortar (up to one order of magnitude).[404] The water content is similar to cement mortar.
6.7.4. Effects upon the Formation of Iron Blue
The first step in the formation of Iron Blue in masonry is the absorption of gaseous hydrogen cyanide. A cool (10°C ) wall in a cellar with atmospheric humidity near the saturation point, due to its higher water content (by a factor of at least 10), has an increased ability (by a factor of 10) to absorb hydrogen cyanide compared to warm walls in a heated room built above ground with lower atmospheric humidity (20°C, 50% rel.).
The second step in the formation of Iron Blue is the ionic split (disassociation) of the hydrogen cyanide, that is, its conversion into simple cyanide.[405] This procedure requires an alkaline environment, which, in lime mortars, lasts only for a few days or weeks, but which are present for months or years in cement mortar and concrete.
The next step is the formation of iron(III)-cyanide, a process that hardly occurs in a strongly alkaline environment and which occurs slowly in slightly alkaline environments. In the neutral range, this reaction is once again slowed down because the cyanide converts into non-reactive, volatile hydrogen cyanide by the humidity in the wall. The environment around the carbonation limit of concrete and mortar (which is slightly alkaline), can therefore be addressed as the area in which iron(III)-cyanide can form easily. In a strongly alkaline area of the masonry, it only arrives at this prior stage of Iron Blue formation through the slow detour of the reduction of slight traces of iron(III)-cyanide to iron(II)-cyanide. A large surface area, as found in cement mortars and concrete, is especially favorable to the solid-liquid interface reaction between solid rust and cyanide in a liquid solution. These generally have the advantage of retaining an alkaline medium for longer periods of time, so that the cyanide accumulated in the masonry is not lost and has enough time to react with rust. Once again, a high water content, which broadens the range of moderately alkaline acid values, is advantageous.[406] The reduction of a part of the iron(III)-ions to iron(II)-ions finally, the next to last step in Iron Blue formation, requires a moderately alkaline acid value, but also occurs in the strongly alkaline range. A distinction can be made between three areas of different reactivity in masonry:
|
Table 7: Absorption of hydrogen cyanide by various building materials under the effect of 2% HCN by volume over 24 hours.[409] |
|
|
Material |
HCN [mg m-2] |
|
Terracotta |
55.2 |
|
Brick |
73.0 |
|
Lime sandstone, naturally humid |
22,740.0 |
|
Lime sandstone, briefly dried |
4,360.0 |
|
Lime sandstone, dried approx. 1/2 year at 20°C |
2,941.0 |
|
Concrete block, dried for 3 days |
8,148.0 |
|
Lime mortar blocks, a few days old* |
4,800.0 |
|
Cement mortar blocks, a few days old* |
540.0 |
|
Cement mortar blocks, a month old* |
140.0 |
|
Cement blocks, pure, a few days old* |
1,550.0 |
|
* 2.5 to 3.3% HCN by volume.[408] The vol. % data, according to the authors, represent theoretical nominal values, which, in practice, however, are only reached up to 50% or less, through adsorption onto walls and fumigation materials. |
|
Table 7 shows the adsorption values of hydrogen cyanide in various building materials.[409] They confirm the assumption of considerably higher reactivity of cements compared to brick, as well as the greater tendency of fresh cement compared to older and generally more humid building materials toward accumulation of hydrogen cyanide. The hydrogen cyanide accumulation in concrete masonry, the age of which is unfortunately not indicated, is astonishingly high. Since by definition there is no considerable difference between the composition of cement mortar and concrete, it is furthermore not clear how the differing analytical results are to be interpreted. These data are therefore not without their difficulties.[410] But at least the tendency of humid masonry to absorb higher quantities of hydrogen cyanide is confirmed (compare lime sandstone: factor 8 at equal temperature and relative atmospheric humidity, but different prior history). W.A. Uglow showed in a detailed series of tests that concrete absorbs approximately four to six times as much hydrogen cyanide as lime mortar. He also found a strong tendency of humid building materials towards increased adsorption of hydrogen cyanide. He also noted a dark blue pigmentation running through the entire concrete sample and did not therefore exclude the possibility of a chemical reaction of the hydrogen cyanide with the material.[411]
The durability of very high concentrations of hydrogen cyanide over longer periods of time even in dry, chemically bound cement may be seen from Graph 8. Concentrations do not fall below 1/4 of the initial values even after three days. With daily fumigation lasting several hours, this resulted, in this example, in average HCN concentration in the wall swinging around approximately 100 to 200 mg hydrogen cyanide per m2 of masonry.
The measurement values in Graph 8 were approximated by a function consisting of two terms:
|
c(t)= 100.e-(t/0.3) + 100.e-(t/4). |
(6) |
|
c(t) = HCN concentration at time t |
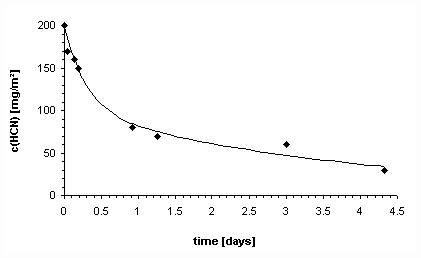 |
|
Graph 8: Drop in the hydrogen cyanide concentration in old, dry, cement blocks, after 24-hour fumigation with 2.5% HCN by volume[409] (see footnote in Table 7, S. 187). |
The first term in the above can be interpreted as desorption from the surface material with a t[412] of 0.3 days. The second term describes a slower desorption of hydrogen cyanide with a t of four days, perhaps caused by the much slower diffusion through the pore water of the samples. Larger errors relating to the drop in concentration described here will be made over longer periods of time because the release of hydrogen cyanide is increasingly inhibited by physical and chemical effects (forming of stable compounds).
An analogous function is assumed by the absorption of hydrogen cyanide:
|
c(t)= 100.(2-e-(t/0.3)-e-(t/4)). |
(7) |
This is only a correct description of the process when the concentration of hydrogen cyanide in air in the room remains constant. The function then reaches its maximum saturation after approximately 20 days. In order to allow for such an approximation, one must reduce the gassing time involved in such a way as to equal real conditions with variable concentrations. In case of a series of consecutive gassings and airings of masonry, a quasi-constant concentration will be reached after 20 cycles as well.
Notes
| [313] | Absorption and Adsorption are not the same! Absorption is the incorporation (sometimes even consumption) of a matter into a medium (light is absorbed/consumed by a pigment, gas is absorbed/dissolves into a liquid), whereas Adsorption is the adhesion of matter onto a-usually solid-surface (dust on furniture, steam on windscreen, vapours on any solid surface...); Adsorption is further subdivided in chemisorption, in which the matter is bound to a surface by chemical bonds, and physisorption, in which the bonding is only a physical effect. The transition between both is fluent. |
| [314] | For simplicity's sake, 'cyanide' is frequently understood to mean only the anionic part of the cyanide salts, the cyanide ion, CN-. |
| [315] | Iron Blue is the ISO designation (ISO 2495) for iron cyanide blue pigments of various composition, which are also known as Berlin Blue, Turnbull's Blue, Prussian Blue, Vossen Blue®, Milori Blue, Paris Blue, French Blue, China Blue, Bronze Blue, Steel Blue, Ink Blue, among others. |
| [316] | Taken from the book by Jürgen Graf, Carlo Mattogno, KL Majdanek, op. cit. (note 43), photos XIII, XIV, XIX (online: www.vho.org/D/Majdanek/MR.html); see also the photo in Michael Berenbaum, The World Must Know, Little, Brown & Co., Boston 1993, p. 138. |
| [317] | Taken from the book by Ernst Gauss (Ed., alias G. Rudolf), Dissecting the Holocaust, op. cit. (note 22), color page, with kind permission by Carlo Mattogno. |
| [318] | Taken from the book by Carlo Mattogno, Jürgen Graf, Das KL Stutthof, op. cit. (note 43), photos 13 & 14 (online: www.vho.org/D/Stutthof). |
| [319] | I also want to point out that I did not include all this academic, self-serving ivory tower chatter in order to impress people. I was simply advised by many friends, supporters, and adversaries to include all my material since back-references to my German original is no help to most English language speakers, of whom only a tiny fraction can read German. |
| [320] | High polarity, low molecular mass, possibility of formation of hydrogen bonds. |
| [321] | Concentration is the number of parts per volume. |
| [322] | W. Braker, A.L. Mossman, Matheson Gas Data Book, Matheson Gas Products, East Rutherford 1971, p. 301. I have left out some of the less interesting dimensions in this connection: heat capacity (20.9°C): 2.625 J g-1 K-1 (Water=4.187 J g-1 K-1); dielectricity constant (20°C): 114 (Water=78.5); evaporation heat: 28 kJ mol-1; evaporation entropy: 190 J mol-1 K-1; spontaneous combustion temperature: 538°C; flash point: -17.8°C; regarding dielectricity constants, see: R.C. Weast (ed.), Handbook of Chemistry and Physics, 66th Ed., CRC Press, Boca Raton, Florida 1986, E 40. However, under normal conditions (1 atm, 25°C), hydrogen cyanide is not a gas. |
| [323] | 1 vol.% is 10,000 ppm (for HCN, roughly 12 g/m³) |
| [324] | The lowered vapor pressure caused by adsorption effects in a narrow hollow space leads to early condensation. |
| [325] | The usual explosive in dynamite. Cf. Wilhelm Foerst (ed.), Ullmanns Encyklopädie der technischen Chemie, vol. 5, Urban und Schwarzenberg, Munich 31954, p. 629. |
| [326] | Willibald Schütz, "Explosionsgefährlichkeit gasförmiger Entwesungsmittel", Reichsarbeitsblatt, Teil III (Arbeitsschutz no. 6), no. 17/18 (1943), pp. 198-207, here p. 201. |
| [327] | Impulseless resonance absorption of g-quants (gamma radiation) from a radioactive isotope, here Cobalt: 57Co à 57Fe + g (main quant: 122 keV; quant used for spectroscopy has a different energy) |
| [328] | E. Fluck, W. Kerler, W. Neuwirth, Z. anorg. allg. Chem. 333 (1964), pp. 235-247; J.F. Duncan, J. Chem. Soc. 1963, pp. 1120-1125. |
| [329] | H.J. Buser, D. Schwarzenbach, W. Peter, A. Ludi, Inorg. Chem. 16 (1977), pp. 2704-2710. Iron Blue single crystals of high purity and homogeneity were obtained by slow oxidation of a solution of Fe[FeII(CN)6] in concentrated (!) HClaq. in air. If in the presence of molar amounts of Kalium only some 2% inclusions were observed. |
| [330] | Originally, this term was used only in organic chemistry for chainlike connected, sometimes also branched attachments of equal segments. |
| [331] | Dispersion (lat.: dispersere, distribute) are distribution of two different phases within each other. They are called colloids (gr.: gluelike) if the particles are between 10-8 and 10-7 m small. Such a mixture in liquids scatters the light (Tyndall effect), is thus not clear. But due to electrostatic repulsion (equally charged particles), colloids do not tend to coagulate and precipitate. Suspension: (lat.: to float) are coarsely dispersed system with particle sizes bigger than 10-6 m. |
| [332] | R.E. Kirk, D.F. Othmer, Encyclopedia of Chemical Technology, Vol. 13, 3. ed., Wiley & Sons, New York 1979, pp. 765-771; J.A. Sistino, in: Peter A. Lewis (ed.), Pigment Handbook, Vol. 1, Wiley and Sons, New York 1974, pp. 401-407; A.F. Holleman, N. Wiberg, Lehrbuch der Anorganischen Chemie, de Gruyter, Berlin 1001985, p. 1143 |
| [333] | H. Ferch, H. Schäfer, Schriftenreihe Pigmente Nr. 77, Degussa AG, Frankfurt 1990. |
| [334] | K.A. Hofmann, Anorganische Chemie, Vieweg, Braunschweig 211973, p. 677; B.N. Gosh, K.C. Ray, Trans. Far. Soc. 53 (1957), pp. 1659-1661; E.F. Zhel'vis, Y.M. Glazman, Ukrainskii Khim. Zh. 35 (1969), pp. 766ff.; East European Sci. Abs. 5 (1969), pp. 84f. |
| [335] | M.B. Robin, Inorg. Chem. 1 (1962), pp. 337-342; Gmelins Handbuch der Anorganischen Chemie, 59 (Fe), B4, Verlag Chemie, Weinheim 1932, pp. 670-732; R.E. Wilde, S.N. Ghosh, B.J. Marshall, Inorg. Chem. 9 (1970), pp. 2512-2516; R.S. Saxena, J. Ind. Chem. Soc. 28 (1951), pp. 703-709; A.K. Bhattacharya, J. Ind. Chem. Soc. 28 (1951), pp. 221-224. |
| [336] | F. Krleza, M. Avlijas, G. Dokovic, Glap. Hem. Tehnol. Bosne Hercegovine, 23-24 (1977, Vol. Date 1976), pp. 7-13. |
| [337] | Photolytic decomposition of the [FeIII(CN)6]3- by means of UV radiation is also conceivable as an alternative. Since the interior walls of the rooms in question are not exposed to any UV radition, this mechanism is ignored here. See also G. Stochel, Z. Stasicka, Polyhedron 4(11) (1985), pp. 1887-1890; T. Ozeki, K. Matsumoto, S. Hikime, Anal. Chem. 56 (14) (1984), pp. 2819-2822; L. Moggi, F. Bolletta, V. Balzani, F. Scandola, J. Inorg. Nucl. Chem. 28 (1966), pp. 2589-2598. |
| [338] | pH value of 9-10 according to M.A. Alich, D.T. Haworth, M.F. Johnson, J. Inorg. Nucl. Chem. 29 (1967), pp. 1637-1642. Spectroscopic studies of the reaction of hexacyanoferrate(III) in water and ethanol. 3.3×10-4 M Fe(NO3)3 were exposed with a cyanide excess of likewise 3.3×10-4 mol l-1. With pH values of approximately 10, all the Fe2[Fe(CN)6] was converted into Iron Blue within 48 hours. Cyanate, the anticipated product of the oxidation of the CN-, could not, however, be proven. Perhaps this is further oxidized directly into CO2. If this mechanism is assumed, the result, purely stoichiometrically, is that an alkaline environment must be favorable. This finding is supported by the known fact that hexacyanoferrate(III) is a strong oxidation agent in alkaline medium and is even able to oxidize trivalent chrome to hexavalent, therefore, that is, CN- ions must have oxidized very quickly: J.C. Bailar, Comprehensive Inorganic Chemistry, Vol. 3, Pergamon Press, Oxford 1973, p. 1047. An overly alkaline environment would, however, disturb the complexing of the Fe3+- ion by cyanide, which is then displaced by OH- (Fe(OH)3 then occurs as a by-product) and/or the latter can hardly be displaced from the iron. The driving force in the reduction of the Fe3+ is the considerably more favorable energetical situation of the hexacyanoferrate(II) as compared to hexacyanoferrate(III); see, in this regard, R.M. Izatt, G.D. Watt, C.H. Bartholomew, J.J. Christensen, Inorg. Chem. 9 (1970), pp. 2019ff. Calorimetric measurements relating to the formation enthalpies of Iron Blue from respective educts (in brackets) were as follows: DH(Fe2+ + [Fe(CN)6]3-)= -66.128 kJ mol-1; DH(Fe3+ + [Fe(CN)6]4-)= 2.197 kJ mol-1. For this reason, a direct reduction of uncomplexed Fe3+, i.e., not surrounded by cyanide, has an energy disadvantage and is therefore negligible. |
| [339] | Dissociation: is the splitting of a compound, in this case into two differently charged ions (heterolytic) in aqueous medium (electrolysis): HCN + H2O à CN- + H3O+ |
| [340] | Correct: hexacyanoferrate(III). |
| [341] | nucleophilic (gr.: core/nucleus loving) is the tendency of a particle to react with positively charged particles. For this, at least a partial negative charge of the nucleophilic particle is required. In this case, cyanide is, due to its negative charge (CN-), much more nucleophilic towards the positively charge iron (Fe3+) than the formally uncharged (though polar) hydrogen cyanide. |
| [342] | pH (pondus hydrogenii) is a measure for the acid content of aqueous solutions (negative, decadic logarithm of H3O+ concentration: -lg10(c(H3O+))): pH < 7: acid; pH = 7: neutral; pH > 7: alkaline |
| [343] | Fe2O(3-x)(OH)2x · x H2O |
| [344] | Naturally, the equilibrium of the reaction Fe(OH)3 + 6 CN- « [Fe(CN)6]3- + 3 OH- under such conditions is strongly on the left hand side. However, this does not mean, as is well known, that a minute quantity of iron(III)-cyanide will not be formed. The latter, however, is withdrawn from the equilibrium in alkaline medium in the presence of excess cyanide, by being reduced by the latter to iron(II)-cyanide, which is considerably more stable in alkaline medium than iron(III)-cyanide; for further details, see also chapter 6.6.1. |
| [345] | In complex chemistry, ligands refer to in most cases negatively charged particles (anions) surrounding in most cases a positively charged central particle (cation, in general a metal ion). In this case, the central atom iron (Fe2+/3+) is surrounded by the ligand cyanide (CN-). |
| [346] | mol is a standard amount of particles: 1 mol = 6.023 × 1023 particles, according to the definition, the number of atoms contained in 12 g Carbon. |
| [347] | Landolt-Börnstein, Eigenschaften der Materie in ihren Aggregatzuständen, part 2, volume b, Lösungsmittelgleichgewichte I, Springer, Berlin 1962, pp. 1-158. |
| [348] | The partial pressure of a gas is it fraction of the total gas content. |
| [349] | The influence is supposed to be based on the fact that carbonic acid (H2CO3, pKA= 6.37) displaces the cyanide ion (CN-) from the equilibrium: 1. CO2 + H2O « H2CO3; 2. CN- + H2CO3 « HCN + HCO3-. Since, however, CO2 is only soluble in water with difficulty and since, in addition, the equilibrium of the reaction CO2 + H2O « H2CO3 (carbonic acid) lies almost completely on the left side, the concentration of carbonic acid in the moisture of masonry itself is several orders of magnitude lower than that of the hydrogen cyanide, even if the content of carbon dioxide in the room under consideration, exposed to hydrogen cyanide, is similar to the hydrogen cyanide content. HCO3-(pKA=10.25), finally, is a weaker acid than HCN (pKA=9.31) and would therefore be displaced by the latter: HCN + HCO3- « CN- + CO2 + H2O. Therefore, even a higher carbon dioxide content in air can hardly influence the absorption of hydrogen cyanide in masonry. |
| [350] | See also J.C. Bailar's remarks on the massive reduction force of Fe(CN)6]3- in the alkaline environment, op. cit. (note 338). |
| [351] | In the immediate vicinity and beyond the freezing point of water, however, the reactivity drops of course. |
| [352] | J. Oudar, Physics and Chemistry of Surfaces, Blackie & Son, Glasgow 1975, pp. 26ff. |
| [353] | R.C. Weast (ed.), op. cit., (note 322), p. D 163. |
| [354] | Protolysis is the splitting of acids (HAc) into their corresponding acid anion (base, Ac-) and proton (H+, or with water to H3O+): HAc + H2O « Ac- + H3O+; here HCN + H2O « CN- + H3O+. |
| [355] | pKA values of HCN: 9.31; R.C. Weast (ed.), op. cit. (note 353). |
| [356] | Valid for ideal solutions. |
| [357] | The hexacyanoferrate acids are very strong acids: J. Jordan, G.J. Ewing, Inorg. Chem. 1 (1962), pp. 587-591. The findings of analyses of disassociation constants show, for hexacyanoferrate(III): K1III>K2III>K3III>0.1; hexacyanoferrate(II): K1II>K2II>0.1; K3II=6×10-3; K4II=6.7×10-5. Thus, hexacyanoferrate(III) is still almost completely disassociated at pH=1, hexacyanoferrate(II) doubly, from pH=3 triply, from pH=5 complete. |
| [358] | G.-O. Müller, Lehrbuch der angewandten Chemie, vol. I, Hirzel, Leipzig 1986, p. 108; the pigment is, however, reversibly soluble in concentrated hydrochloric acid, i.e., the pigment is not decomposed, but merely physically brought into solution; there is therefore no release of hydrogen cyanide; see also H.J. Buser et al., op. cit. (note 329); see also chapter 8.2.: analytical method for total cyanide content according to DIN: the pigment is destroyed by boiling HClaq.. Iron Blue suspensions (see note 331) have an acid pH value of approximately 4. At this slightly acid eigen pH, as is formed, for example, by acid rain in surface waters, Iron Blue is at its most stable: H. Ferch, H. Schäfer, op. cit. (note 333). In technical applications, the alkaline resistance is increase by adding nickel, cf. R.E. Kirk, D.F. Othmer, op. cit. (note 332); J.A. Sistino, op. cit. (note 332); E. Elsermann, Deutsche Farben-Z. 5 (1951), pp. 419ff.; R. Beck, Deutsche Farben-Z. 6 (1952), p. 231. |
| [359] | Iron(III)-hydroxide is even less soluble in this range than Iron Blue; on the solubility of Fe(OH)3 see chapter 6.6.3.; to be exact, Iron Blue is not totally destroyed at a high pH; rather, the Fe3+ is, initially, merely withdrawn; the base-resistant [Fe(CN)6]4- remains intact; see note 344. |
| [360] | See the studies by M.A. Alich et al., op. cit. (note 338). |
| [361] | J.A. Sistino, op. cit. (note 332); H. Beakes, Paint Ind. Mag. 69(11) (1954), pp. 33f. Mixtures of Iron Blue and phtalocyanine blue generally find application, since both, alone, lack sufficient long-term stability; Degussa describes the lime fastness of Iron Blue as "not good" (H. Ferch, H. Schäfer, op. cit. (note 333)); however, Degussa is referring to its fastness on still uncarbonated, alkaline plasters and concretes: H. Winkler, Degussa AG, letter to this author, June 18, 1991. My own experiments with the dissolution of fresh Iron Blue precipitations resulted in a limit value of pH 10-11 for the stability of Iron Blue. |
| [362] | This property is used in Russian industry, for example, for the passivation of steel pipes against aggressive waste waters, since CN- contained in waste waters coats the interior of pipes with an insoluble protective layer of Iron Blue: N.G. Chen, J. Appl. Chem. USSR, 74(1)(1974), pp. 139-142. But it should be noted that this borders on criminal negligence, since toxic cyanides simply do not belong in waste waters. |
| [363] | DIN Safety Data Sheet VOSSEN-Blau®, in: Schriftenreihe Pigmente Nr. 50, Degussa AG, Frankfurt 1985; see also H. Ferch, H. Schäfer, op. cit. (note 333). Last but not least, pigments, by definition, are coloring agents practically insoluble in dissolvents and binding agents (DIN 55,943 and 55,945). |
| [364] | See also, in this regard, the remarks of a company dealing in colored cements and concretes: Davis Colors, 3700 East Olympics Blvd., Los Angeles, CA 90023, www.coloredconcrete.com/davis/Tech/03470.html. |
| [365] | I.V. Tananaev, M.A. Glushkova, G.B. Seifer, J. Inorg. Chem. USSR, 1 (1956), pp. 72ff. |
| [366] | The solubility product of a compound is defined as the product of the entire ionic concentration of the totally dissociated compound: Fe4[Fe(CN)6]3 ® 4 Fe3+ + 3 [Fe(CN)6]4-; KL(Fe4[Fe(CN)6]3) = c(Fe3+)·c(Fe3+)·c(Fe3+)·c(Fe3+)·c([Fe(CN)6]4-)·c([Fe(CN)6]4-)·c([Fe(CN)6]4-) = c4(Fe3+)·c3([Fe(CN)6]4-). The pKS value correlates to the negative decimal logarithm of the product of solubility. |
| [367] | R.C. Weast (ed.), op. cit., (note 322), p. B 222. |
| [368] | C. Wilson, Wilson & Wilson's Comprehensive Inorganic Chemistry, Vol. 1B, Elsevier, Amsterdam 1960, p. 162. |
| [369] | In absence of free cyanide ions, the pH stability limit of hexacyanoferrate(II) (total dissociation) is at 11.8, but already very small amounts of free cyanide (10-10 mol l-1) push the limit up to pH=13. |
| [370] | Tartrate, corresponding base of tartaric acid. The mixed potassium-sodium-salt is the famous tartrate (potassium bitartrate), which crystallizes on the cork of wine bottles (Seignette salt). |
| [371] | C. Lapp, C. Wehrer, P. Laugel, Analusis, 13 (4) (1985), pp. 185-190. |
| [372] | G.-O. Müller, op. cit. (note 358). |
| [373] | M. Kohn, Anal. Chim. Acta 3 (1949), pp. 558ff.; ibid., 5 (1951), pp. 525-528; ibid., 11 (1954), pp. 18-27. |
| [374] | See also Winnacker-Küchler, Chemische Technologie, volume 2, Carl Hanser Verlag, Munich 1982, p. 197; H. Ferch, H. Schäfer, op. cit. (note 333); Wilhelm Foerst (ed.), Ullmanns Encyklopädie der technischen Chemie, volume 13, Urban und Schwarzenberg, Munich 31962, p. 794; ibid., volume 18, Verlag Chemie, Weinheim 1979, pp. 623ff.; H. Watanabe, J. Jap. Soc. Col. Mat., 34 (1961), pp. 5-8; L. Müller-Focken, Farbe und Lack, 84 (1987), pp. 489-492. |
| [375] | H. Tada, M. Kunio, H. Kawahara, Jpn. Kokai Tokkyo Koho, 1990, 3 p. Source only available as abstract. |
| [376] | Quantum efficiency is that part of the absorbed light quants which leads to photo reactions under scrutiny, here from 10 to 40%. |
| [377] | L. Moggi, et al., op. cit. (note 337); V. Carassiti, V. Balzani, Ann. Chim. 50 (1960), pp. 782-789. |
| [378] | Photolysis of water leads to the splitting of water into uncharged parts with unpaired electrons (formation of radicals through homolytic splitting (homolysis); see also dissociation, note 339): 2 H2O + hn à H3O· + OH·(hydroxyl radical; hn = photo quant) |
| [379] | M.D. Gurol, J.H. Woodman, Hazard. Ind. Waste 21 (1989), pp. 282-290; S.A. Zaidi, J. Carey, in: Proceedings of the Conference on Cyanide and the Environment, Colorado State University, 1984, pp. 363-377. |
| [380] | Deutsche Chemische Gesellschaft (ed.), Gmelins Handbuch, op. cit. (note 335); Ullmanns Encyklopädie, op. cit. (note 374); L. Müller-Focken, op. cit. (note 374). |
| [381] | J.M. Kape, E.C. Mills, Tranp. Inst. Met. Finish., 35 (1958), pp. 353-384; ibid., 59 (1981), pp. 35-39. |
| [382] | K4[Fe(CN)6]3 or Fe(NO3)3. |
| [383] | The literature does not, however, mention this Iron Blue sample as "Prussian Blue", like the others, since it was, at that time, considered to be of another type, i.e., "Turnbull's Blue" or "ferrous ferricyanide". |
| [384] | Compare Ferch, H. Schäfer, op. cit. (note 333); S. Barbezat, J. Réch. Cent. Nat. Réch. Sci. 4 (1952), pp. 184ff.; E. Gratzfeld, Färg och Lack, 3 (1957), pp. 85-108; E. Herrmann, Farbe und Lack, 64 (1958), pp. 130-135. |
| [385] | D. Maier, K. Czurda, G. Gudehus, Das Gas- und Wasserfach, in: Gas • Erdgas, 130 (1989), pp. 474-484. |
| [386] | An interesting study has been conducted in this connection about the reduction of soluble components in concrete standing in water, providing support to the statements made here: not even the concentration of alkali ions, which are the most soluble components of concrete, was massively reduced: H.A. El-Sayed, Cement and Concrete Research, 11 (1981), pp. 351-362. |
| [387] | German daily newspapers, for instance: Süddeutsche Zeitung, Stuttgarter Zeitung, Südwestpresse-Verbund (March 29, 1994), taz, Frankfurter Rundschau (March 30, 1994). |
| [388] | See also W. Schlesiger, op. cit. (note 91), pp. 21-24; G. Rudolf, "Über die frei erfundene Expertenmeinung der 'dpa'", DGG 42(2) (1994), pp. 25f. (online: www.vho.org/D/DGG/Rudolf42_2.html); Engl. in the appendix to this book, chapter 11.5. |
| [389] | See the Bavarian State Ministry for the Interior, Verfassungsschutzbericht 1997, Munich 1998, p. 64. A corresponding reference to the factual incorrectness of the remarks made in this regard by the Arbeitskreis Zeitgeschichte und Politik (in a letter by president Hans-Jürgen Witzsch, dated Oct. 8, 1998, Fürth) was countered by the Ministry as follows: "Your efforts to deny and/or relativize the crimes of the National Socialists have been known to the security authorities for years. [...] We see no occasion for a discussion of gas chambers." The letter, from Dr. Weber of the Bavarian State Ministry of the Interior dated Oct. 13, 1998, ref. IF1-1335.31-1, probably established a new world record for stupidty. |
| [390] | O. Hähnle, Baustoff-Lexikon, Deutsche Verlagsanstalt, Stuttgart 1961, p. 384. |
| [391] | Landolt-Börnstein, Zahlen und Funktionen aus Physik, Chemie, Astronomie, Technik, volume IV Technik, part 4b Wärmetechnik, Springer, Berlin 61972, pp. 433-452. |
| [392] | S. Röbert (ed.), Systematische Baustofflehre, volume 1, VEB Verlag für Bauwesen, Berlin 41983, p. 120. |
| [393] | These mercury penetration tests were performed at the research institute of the VARTA Batterie AG in Kelkheim, Germany, in late 1991. |
| [394] | Method to determine the specific surface with nitrogen adsorption following Brunauer, Emmet, Teller. |
| [395] | K. Wesche, Baustoffe für tragende Bauteile, volume 1, Bauverlag, Wiesbaden 1977, p. 37. |
| [396] | W.H. Duda, Cement-Data-Book, Bauverlag, Wiesbaden 1976, pp. 4ff., as well as my own analysis. |
| [397] | W. Czernin, Zementchemie für Bauingenieure, Bauverlag, Wiesbaden 1977, pp. 49f. |
| [398] | N.V. Waubke, Transportphänomene in Betonporen, Dissertation, Braunschweig 1966. |
| [399] | In the strongly alkaline environment, iron is passivated by a passive layer of Fe(OH)3. 'Botch work' on building sites, i.e., rusting reinforcement rods and cracking concrete after only a few years or decades, due to overly low pH value in the vicinity of the embedded reinforcement rods, is caused by a) an incorrect composition of the concrete (too little cement-it's cheaper this way-and/or too much or too little water-incompetence), or b) by installing the reinforcement rods too close to the surface of the concrete, where the pH value falls strongly after a few years or decades; see notes 396f. |
| [400] | K. Wesche, Baustoffe für tragende Bauteile, volume 2, Bauverlag, Wiesbaden 1981, pp. 51f. |
| [401] | Verein Deutscher Zementwerke, Zement Taschenbuch 1972/73, Bauverlag, Wiesbaden 1972, pp. 19ff. |
| [402] | W.H. Duda, op. cit. (note 396). |
| [403] | W. Czernin, op. cit. (note 397); Verein Deutscher Zementwerke, op. cit. (note 401); N.V. Waubke, op. cit. (note 398). |
| [404] | The reason: no formation of very finely crystalline alumosilicate with higher surface area. |
| [405] | In masonry, this largely corresponds to the neutralization of the hydrogen cyanide by calcium hydroxide Ca(OH)2 into calcium cyanide Ca(CN)2. |
| [406] | Very humid mortars and concretes, due to proton diffusion, exhibit no sharp carbonation, i.e., pH limit. |
| [407] | From the CO2 in the air and the Ca(OH)2 in the mortar. |
| [408] | F. Puntigam, et al., op. cit. (note 122), pp. 35ff. |
| [409] | L. Schwarz, W. Deckert, Z. Hygiene und Infektionskrankheiten, 107 (1927), pp. 798-813; ibid., 109 (1929), pp. 201-212. |
| [410] | The method cannot establish any possible chemical binding of hydrogen cyanide, since only that fraction of hydrogen cyanide was measured which evaporated from the samples. |
| [411] | W.A. Uglow, Z. Hygiene und Infektionskrankheiten, 108 (1928), pp. 108-123. |
| [412] | t is the time after which the value has fallen to the 1/e-multiple (0.368...) of the initial value. |
Next Chapter
Previous Chapter
Back to Table of Contents